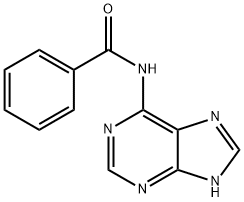
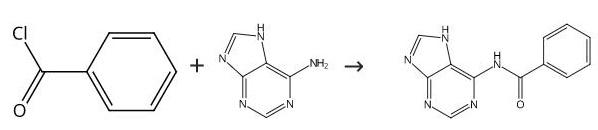
N-Benzoylaminopurine synthesis
- Product Name:N-Benzoylaminopurine
- CAS Number:4005-49-6
- Molecular formula:C12H9N5O
- Molecular Weight:239.23
![Benzamide, N-[9-[3-C-ethenyl-3,5-O-(1-methylethylidene)-β-D-xylofuranosyl]-9H-purin-6-yl]-](/CAS/20210305/GIF/188486-36-4.gif)
188486-36-4
0 suppliers
inquiry

4005-49-6
250 suppliers
$11.00/5g
Yield:-
Steps:
Multi-step reaction with 6 steps
1.1: 97 percent / pyridine / 0 - 20 °C
2.1: ozone / CH2Cl2 / 1 h / -78 °C
2.2: 69 percent / Me2S / CH2Cl2 / 1 h / -78 - 20 °C
3.1: 91 percent / KMnO4; phosphate buffer / 2-methyl-propan-2-ol; H2O / 0.5 h / 20 °C / pH 7
4.1: NEt3; phenyl dichlorophosphate / tetrahydrofuran / 1 h / 0 °C
4.2: 72 percent / NEt3 / tetrahydrofuran / 0.5 h / 0 °C
5.1: 86 percent / trifluoroacetic acid; water / 0.5 h / 20 °C
6.1: 84 percent Spectr. / Bu3SnH / methanol / 3 h / 20 °C / Photolysis
References:
Lenz, Roman;Giese, Bernd [Journal of the American Chemical Society,1997,vol. 119,# 12,p. 2784 - 2794]
![Adenosine, 2',5'-bis-O-[(1,1-dimethylethyl)dimethylsilyl]-](/CAS/20200611/GIF/65109-11-7.gif)
65109-11-7
0 suppliers
inquiry

4005-49-6
250 suppliers
$11.00/5g
![Benzamide, N-[9-(3-C-ethenyl-β-D-xylofuranosyl)-9H-purin-6-yl]-](/CAS/20210305/GIF/188486-35-3.gif)
188486-35-3
0 suppliers
inquiry

4005-49-6
250 suppliers
$11.00/5g