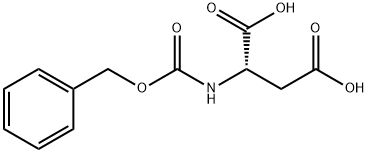
N-Carbobenzyloxy-L-aspartic acid synthesis
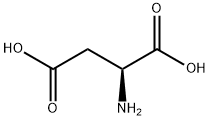
- Product Name:N-Carbobenzyloxy-L-aspartic acid
- CAS Number:1152-61-0
- Molecular formula:C12H13NO6
- Molecular Weight:267.23
Yield:1152-61-0 94%
Reaction Conditions:
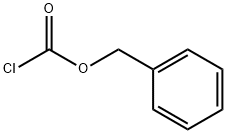
Stage #1:L-Aspartic acid;benzyl chloroformate with potassium carbonate in water at 0 - 20; for 16 h;
Stage #2: with hydrogenchloride in water; pH=1
Steps:
1.1-1(1)
103.8 g (751 mmol) of potassium carbonate was dissolved in 1 L of distilled water, and 50.0 g (376 mmol) of aspartic acid was dissolved therein. The solution was cooled to temperatures ranging from 0 to 5°C, and then 74.1 mL (526 mmol) of benzyl chloroformate was slowly added dropwise. The reactant was stirred at room temperature for 16 hours, and washed with diethyl ether. An aqueous layer was adjusted to pH 1 with 6 N hydrochloric acid. The pH-adjusted aqueous layer was extracted with ethyl acetate several times. An organic layer was dried over magnesium sulfate, and concentrated under vacuum to give 94.4 g of highly viscous liquid concentrate (yield: 94%). The next step was performed without further purification.
References:
DAEWOONG PHARMACEUTICAL CO., LTD.;CHOI, Soo Jin;LEE, Byung Goo;OH, Seong Soo;KIM, Yong Tae;EO, Jin Yong;KIM, Hung Sop WO2012/148246, 2012, A2 Location in patent:Page/Page column 12
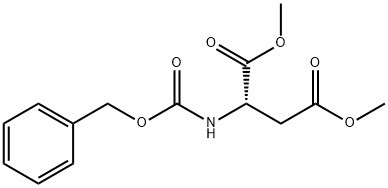
35909-93-4
9 suppliers
inquiry

1152-61-0
350 suppliers
$5.00/1g
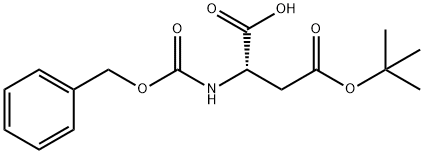
5545-52-8
216 suppliers
$11.19/1G

1152-61-0
350 suppliers
$5.00/1g