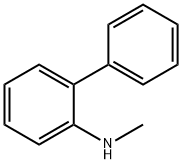
N-methylbiphenyl-2-amine synthesis
- Product Name:N-methylbiphenyl-2-amine
- CAS Number:14925-09-8
- Molecular formula:C13H13N
- Molecular Weight:183.25
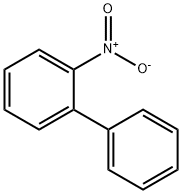
86-00-0
133 suppliers
$11.00/1g

124-38-9
122 suppliers
$175.00/23402

14925-09-8
97 suppliers
$32.00/100mg
Yield: 83%
Reaction Conditions:
with hydrogen in hexane at 170; under 52505.3 Torr; for 48 h;Autoclave;
Steps:
80
General procedure: The procedures were same as that in Example 19. Ina 100 mE reaction autoclave with magnetic stirring, 50 mgCatalyst A was used. The reaction medium was 2 mE n-hexane. 1 mmol starting material was added respectively. After being sealed, the inside of the autoclave was replaced by carbon dioxide gas for three times. Then carbon dioxide gas and hydrogen gas were charged, wherein the starting material, the pressure of the autoclave afier charging carbon dioxide and hydrogen gas (02 and H2’ respectively), reaction temperature, reaction time and target products (qualitative analysis and detection were similar to those in Example 46) were shown in Table 2 below, respectively. After stopping the reaction and cooling to RT, the reaction mixture was subjected to a quantitative analysis withAgilent 7890A gas-mass chromatograph (30 mx0.25 mmx0.33 tm capillary column, with a hydrogen flame ionization detector). The target products were obtained by the conventional separation and purification methods well known in the art, such as rectification.The yields ofthe target products were shown in Table 2 below.
References:
Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences;SHI, Feng;Cui, Xinjiang;Yuan, Hangkong;Zhang, Yan;Deng, Youquan US2015/18548, 2015, A1 Location in patent:Paragraph 0059
1484-12-4
94 suppliers
$16.00/1g

14925-09-8
97 suppliers
$32.00/100mg

50-00-0
838 suppliers
$10.00/25g
90-41-5
344 suppliers
$16.00/5g

14925-09-8
97 suppliers
$32.00/100mg
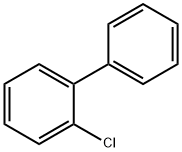
2051-60-7
110 suppliers
$21.00/1g
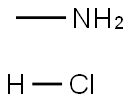
593-51-1
528 suppliers
$21.00/100g

14925-09-8
97 suppliers
$32.00/100mg
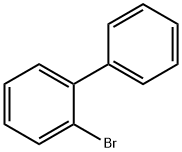
2052-07-5
385 suppliers
$16.30/1g

593-51-1
528 suppliers
$21.00/100g

14925-09-8
97 suppliers
$32.00/100mg