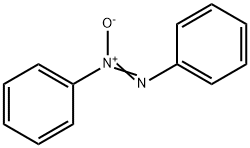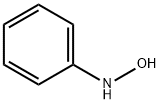
N-Phenylhydroxylamine synthesis
- Product Name:N-Phenylhydroxylamine
- CAS Number:100-65-2
- Molecular formula:C6H7NO
- Molecular Weight:109.13
The reduction of 2-methyl-2-nitropropane is carried out in a similar manner at 10-20°C to afford a 68% yield of N-t-butylhydroxylamine (m.p. 60-62°C).
98-95-3
7 suppliers
$14.00/25g

100-65-2
104 suppliers
$76.00/1 g
Yield:100-65-2 98%
Reaction Conditions:
with hydrogen;silica-supported platinum;dimethyl sulfoxide;N-butylamine in isopropyl alcohol at 20; under 760.051 Torr; for 1.75 h;Product distribution / selectivity;
Steps:
51
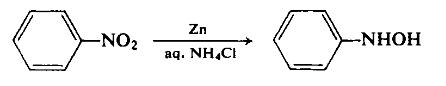
Into a flask, 20 mg of the silica gel platinum catalyst (5% by weight of Pt) [product name: 5% Pt-silica powder "Escat 2351", manufactured by STREM Co., Ltd.] (0.0026 time mole of platinum, relative to nitrobenzene), 2 mL of IPA (isopropyl alcohol), 0.42 mmol of dimethylsulfoxide (30 µL), 0.072 mmol of n-butylamine (7.1 µL) (14 equivalents, relative to platinum weight in the silica gel platinum catalyst) and 2 mmol of various nitroaryl compounds described in the following Table 5 were charged, and then a balloon sealed with hydrogen gas (about 2 L) was attached, and a reaction was carried out under stirring at room temperature for predetermined time under ordinary pressure. After completion of the reaction, 20 µL of the resultant reaction solution was diluted with 10 mL of 2-propanol containing 0.1 M of toluene, and after removal of the catalyst by using a membrane filter, IPA was removed under reduced pressure, and it was analyzed with NMR. The results are summarized in Table 5.
References:
Wako Pure Chemical Industries, Ltd.;National Institute of Advanced Industrial Science and Technology EP2154125, 2010, A1 Location in patent:Page/Page column 23-24

62-53-3
620 suppliers
$10.00/1g

100-65-2
104 suppliers
$76.00/1 g
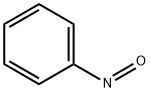
586-96-9
119 suppliers
$20.00/1G

100-65-2
104 suppliers
$76.00/1 g