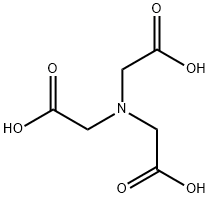
Nitrilotriacetic acid synthesis
- Product Name:Nitrilotriacetic acid
- CAS Number:139-13-9
- Molecular formula:C6H9NO6
- Molecular Weight:191.14
Chloroacetic acid is reacted with sodium hydroxide to produce sodium chloroacetate.
Sodium chloroacetate then reacts with ammonium chloride to form sodium aminotriacetate.
The resulting sodium aminotriacetate is acidified to obtain nitrilotriacetic acid.
Yield:-
Reaction Conditions:
with hydrogenchloride;palladium-carbon in sodium hydroxide;water
Steps:
4 Synthesis of "Nitrilotriacetic Acid" (NTA)
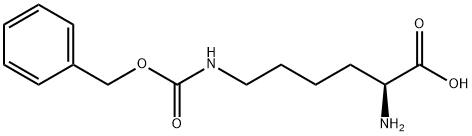
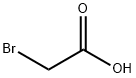
Example 4 Synthesis of "Nitrilotriacetic Acid" (NTA) N,N-Bis-(carboxymethyl)lysine (commonly referred to as "Nitrilotriacetic acid," or "NTA") was synthesised as follows based the procedure reported by Hochuli et al. (Journal of Chromatography, 411:177-184 (1987)). A solution of H-Lys(Z)-OH (42 g; 150 mmol) in 2N NaOH (225 mL) was added drop wise to a solution of bromoacetic acid (42 g; 300 mmol; 2 eq) in 2N NaOH (150 mL) at ~0 to 10° C. White precipitate formed as the solution of H-Lys(Z)-OH added. The reaction continued at room temperature (RT) overnight, after which the temperature was increased to 60° C. and the reaction continued for another 2 h. 1N HCl (450 mL) was added and the mixture was place in a refrigerator for a couple hours. The solid product (Z-protected NTA) was filtered off and recrystallized by re-dissolving the solid in 1N NaOH, then neutralized with the same amount of 1N HCl. The Z-protected NTA was collected by filtration and dried. Z-protected NTA was dissolved in 1N NaOH (130 mL) and 5% Pd/C (~450 mg) was added. The reaction mixture was evacuated and saturated with H2 before being stirred at RT under H2 balloon overnight. The reaction mixture was filtered through a celite bed to remove the Pd/C. The filtrate, containing NTA was collected and water (80 mL) was used to wash the filtering bed. 6N HCl was added to bring the pH down to 7.5-8.0. The collected NTA solution was diluted with water to have the final concentration of ~200 mM.
References:
Gjerde, Douglas T.;Hanna, Christopher T. US2004/241721, 2004, A1
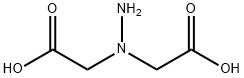
19247-05-3
7 suppliers
inquiry

139-13-9
439 suppliers
$10.00/100G
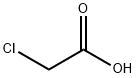
79-11-8
10 suppliers
$24.00/25g

139-13-9
439 suppliers
$10.00/100G