O-ACETYL-L-HOMOSERINE HYDROCHLORIDE synthesis
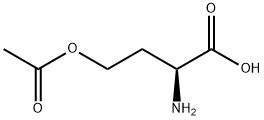
- Product Name:O-ACETYL-L-HOMOSERINE HYDROCHLORIDE
- CAS Number:7540-67-2
- Molecular formula:C6H11NO4
- Molecular Weight:161.16
Yield:7540-67-2 61%
Reaction Conditions:
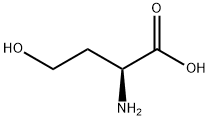
Stage #1: L-homoserinewith perchloric acid in acetic acid at 16 - 18; for 1.5 h;
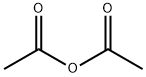
Stage #2: acetic anhydride in acetic acid at 20; for 1.5 h;
Steps:
3.2. Synthesis of O-Acetyl-L-homoserine
To a round bottom flask containing a mixture of glacial acetic acid (99.9%, 20.0 mL, 345.9 mmol)and perchloric acid (60%, 1.7 mL, 43.3 mmol) cooled at 16-18 °C, was carefully added acetic anhydride(99%, 8.1 mL, 84.8 mmol) under stirring, followed by the addition of L-homoserine (1.0 g, 8.4 mmol)previously dissolved in acetic acid (10.0 mL). The reaction was allowed to reach room temperature andstirred for 90 min. Unreacted acetic anhydride was then quenched by addition of water (0.22 mL) andthe mixture was neutralized with pentylamine (2.0 mL, 17.2 mmol). After adding diethyl ether(300 mL), the crude was kept overnight at 4 °C and then five more hours at -20 °C. A precipitate ofhomoserine lactone was formed and removed by filtration. Filtrate was diluted with water (15 mL),filtered again, and finally diluted in EtOH. After standing overnight at 4 °C, a second precipitate wasobtained. This product was isolated by filtration and recrystallized twice from EtOH to give 0.82 g(5.1 mmol, 61% yield) of O-acetyl-L-homoserine as a white solid.
References:
Ma, Ying;Biava, Hernan;Contestabile, Roberto;Budisa, Nediljko;Di Salvo, Martino Luigi [Molecules,2014,vol. 19,# 1,p. 1004 - 1022]