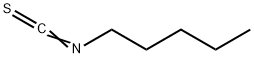
PENTYL ISOTHIOCYANATE synthesis
- Product Name:PENTYL ISOTHIOCYANATE
- CAS Number:629-12-9
- Molecular formula:C6H11NS
- Molecular Weight:129.22
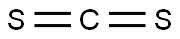
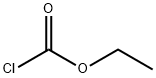
75-15-0
233 suppliers
$40.00/100 g

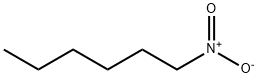
110-58-7
203 suppliers
$14.00/25g

629-12-9
55 suppliers
$43.00/1 g
Yield: 85%
Reaction Conditions:
Stage #1:carbon disulfide;n-Pentylamine with triethylamine in N,N-dimethyl-formamide at 0;
Stage #2: with O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate in N,N-dimethyl-formamide at 0; for 0.25 h;
Steps:
General synthesis of diisothiocyanates
General procedure: Diamine (1 eq) was added dropwise to a solution of carbon disulfide (20 eq) and triethylamine (2eq) in DMF at 0 °C and stirred for 15 min. Subsequently, 1.5 eq of HBTU was added to the reaction mixture and the temperature was maintained at 0 °C for the following 15 min. The reaction mixture was diluted with distilled water (6:1, H2O:DMF) and extracted 3-times with hexane. Combined organic fractions were washed with brine, dried over anhydrous MgSO4 and evaporated to dryness. The crude product was purified by column chromatography with hexane/EtOAc (1:1, v/v) as an eluent. Compound 25: pentyl isothiocyanate; yield 85 %; 1HNMR (300 MHz, CDCl3): δ 3.52 (t, J = 6.6, 2H), 1.81 - 1.62 (m, 2H), 1.48 - 1.27 (m, 4H), 0.92 (t, J = 7.1 Hz, 3H); HRMS: observed m/z, 130.0698, calculated for C6H11NS, 130.0690 [M+ H]+.
References:
Grzywa, Renata;Winiarski, Łukasz;Psurski, Mateusz;Rudnicka, Agata;Wietrzyk, Joanna;Gajda, Tadeusz;Oleksyszyn, Józef [Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,# 2,p. 667 - 671] Location in patent:supporting information

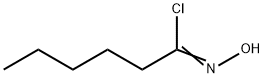
18971-59-0
9 suppliers
$78.50/1g

629-12-9
55 suppliers
$43.00/1 g