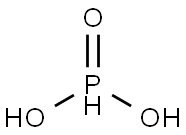
Phosphorous acid synthesis
- Product Name:Phosphorous acid
- CAS Number:13598-36-2
- Molecular formula:H3O3P
- Molecular Weight:82

7732-18-5
451 suppliers
$13.50/100ML
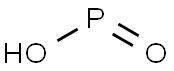
6303-21-5
295 suppliers
$35.50/50g

13598-36-2
313 suppliers
$25.00/10g
Yield:13598-36-2 84%
Reaction Conditions:
with Ni-doped silica for 18 h;Reflux;
Steps:
0056] Phosphorous acid: (Table 2, entry 12). After concentration of water, 1.72 g (84%) of clear liquid was obtained. 1H NMR (400 MHz, CDCl3) δ 6.72 (d, 1JHP=663.2 Hz, 1H); 31P NMR (161.97 MHz, CDCl3) δ 4.37 (d, JPH=664 Hz).
References:
US2014/303394,2014,A1 Location in patent:Paragraph 0036; 0056

6303-21-5
295 suppliers
$35.50/50g

13598-36-2
313 suppliers
$25.00/10g
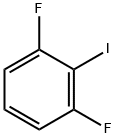
13697-89-7
154 suppliers
$9.00/5g

13598-36-2
313 suppliers
$25.00/10g