
S-3-N-Cbz-amino-2,6-Dioxo-piperidine synthesis
- Product Name:S-3-N-Cbz-amino-2,6-Dioxo-piperidine
- CAS Number:22785-43-9
- Molecular formula:C13H14N2O4
- Molecular Weight:262.26
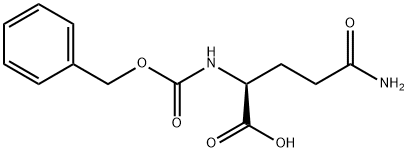
2650-64-8
350 suppliers
$10.00/5g

22785-43-9
45 suppliers
$60.00/250mg
Yield:22785-43-9 90%
Reaction Conditions:
with 1,1'-carbonyldiimidazole in tetrahydrofuran; for 18 h;Reflux;Time;Temperature;Reagent/catalyst;
Steps:
6.2 6.2 Preparation of 4-amino-2-(2,6-dioxopiperidin-3-yl)-1H-isoindole- 1,3-dione (pomalidomide)
Into a stirring solution of carboxybenzyloxy-L-glutamine (2.8 g, 10 mmols) in 40 mL anhydrous THF, 1,1-carbonyldiimidazole (1.92 g, 12 mmols) were added. The reaction mixture was heated under reflux for 18 hours. The THF was evaporated and the product was dissolved in chloroform. The chloroform layer was washed with water and brine and dried over anhydrous CaSO4, filtered and evaporated to give white solid. The solid product was crystallized from ethyl ether to give 2.4 grams crystalline powder (90%). (Alternatively, carboxybenzyloxy-L-glutamine can be cyclized by treating with SOCl2 in Ν,Ν-dimethylformamide at -70°C to 0°C for 1 hour to form the product). The reaction mixture was diluted with CHCI3 and washed with 5% Na2CO3, dried over anhydrous Na2SO4, filtered, and evaporated to give 2.5 g (90% yield) S(-)-(3- benzyloxycarbonylamino)-glutarimide). 1H NMR (CDCl3) δ 8.2 (1H, s broad), 7.4 (5H, s, aromatic), 5.8 (1H, d), 5.15 (2H, s), 4.4 (1H, dd, J=4.5, 3), 2.95-2.4 (3H, m), 1.86 (1H, d, t, J=11.5, 6.5). m.p. 122-124°C (lit. 122-124°C)
References:
WO2014/39960,2014,A1 Location in patent:Page/Page column 57
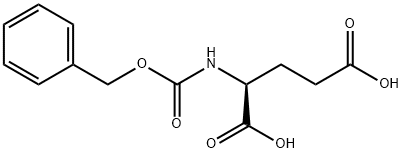
1155-62-0
353 suppliers
$10.00/5g

22785-43-9
45 suppliers
$60.00/250mg
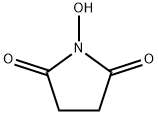
6066-82-6
729 suppliers
$5.00/10g

2650-64-8
350 suppliers
$10.00/5g

34078-85-8
39 suppliers
$40.00/1G

22785-43-9
45 suppliers
$60.00/250mg
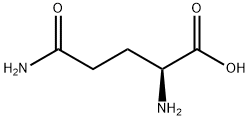
56-85-9
986 suppliers
$5.00/5g

501-53-1
393 suppliers
$10.00/1g

22785-43-9
45 suppliers
$60.00/250mg