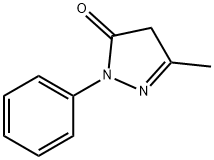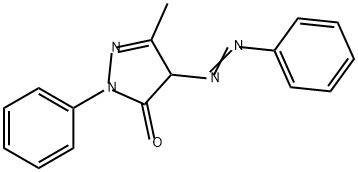
Solvent Yellow 16 synthesis
- Product Name:Solvent Yellow 16
- CAS Number:4314-14-1
- Molecular formula:C16H14N4O
- Molecular Weight:278.31
Yield:-
Reaction Conditions:
Stage #1: anilinewith hydrogenchloride;sodium nitrite in water at 5;
Stage #2: edaravonewith sodium carbonate in water; for 0.2 h;Cooling;Concentration;Time;
Steps:
2; 5 Example 2: Synthesis Method for Solvent Yellow 16
1 gm of aniline was dispersed in 5 mL water in a jacketed batch reactor maintained at 5 °C. 2.86 mL of 35% hydrochloric acid was added to the suspension and the solution was cooled to 5 °C. 0.97 gm of sodium nitrite was dissolved in 7 mL of water and precooled to 5 °C. This sodium nitrite solution was added to the reactor in a drop wise manner to maintain isothermal condition to generate diazonium salt. 1.96 gm of 3 -Methyl- 1 -phenyl- lH-pyrazol-5(4H)-one and 2.27 gm of sodium carbonate was dissolved in 32.5 mL of water and precooled and was added to diazonium salt solution. The reaction was complete in approximately 12 minutes. The product was filtered and dried. The isolated yield was in the range of 97%. Figure 7(a-b) shows the diazotization and coupling reaction for the solvent yellow 16 dye.
References:
WO2021/95059,2021,A1 Location in patent:Paragraph 0052; 0057