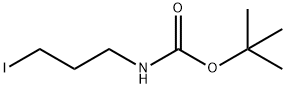
TERT-BUTYL (3-IODOPROPYL)CARBAMATE synthesis
- Product Name:TERT-BUTYL (3-IODOPROPYL)CARBAMATE
- CAS Number:167479-01-8
- Molecular formula:C8H16INO2
- Molecular Weight:285.12
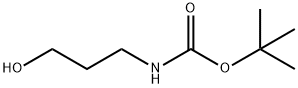
58885-58-8
180 suppliers
$10.00/1g
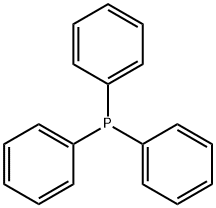
603-35-0
687 suppliers
$5.00/5 g
791-28-6
483 suppliers
$6.00/25g

167479-01-8
40 suppliers
$65.00/250mg
Yield:-
Reaction Conditions:
with 1H-imidazole;iodine in diethyl ether;acetonitrile at 0 - 20;
Steps:
4.B
A mixture of tert-bntyl 3-hydroxypropylcarbamate (19.36 g, 110.5 mmol), triphenylphosphine (34.76 g, 132.6 mmol), imidazole (10.53 g, 154.7 mmol), diethyl ether EPO
References:
3M INNOVATIVE PROPERTIES COMPANY WO2006/86449, 2006, A2 Location in patent:Page/Page column 88-89

58885-58-8
180 suppliers
$10.00/1g

167479-01-8
40 suppliers
$65.00/250mg
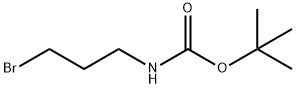
83948-53-2
194 suppliers
$6.00/1g

167479-01-8
40 suppliers
$65.00/250mg

288-32-4
970 suppliers
$5.00/25g

58885-58-8
180 suppliers
$10.00/1g

167479-01-8
40 suppliers
$65.00/250mg

24424-99-5
813 suppliers
$13.50/25G

167479-01-8
40 suppliers
$65.00/250mg