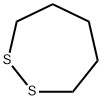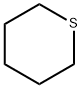
Thiane synthesis
- Product Name:Thiane
- CAS Number:1613-51-0
- Molecular formula:C5H10S
- Molecular Weight:102.2

111-24-0
384 suppliers
$5.00/5g

1613-51-0
90 suppliers
$25.39/1g
Yield:1613-51-0 80%
Reaction Conditions:
with sodiumsulfide nonahydrate at 170; for 7 h;
Steps:
4.2. Typical procedure for the preparation of cyclic sulfides 1-4
General procedure: A mixture of the appropriately substituted 1,5-dibromopentane and sodium sulfide nonahydrate(for quantities, see individual compound sections) was heated at 170°C for 7 h inan oil bath. After cooling, water (20ml) and dichloromethane (DCM, 20ml) were added. The phases were separated and the aqueous layer was re-extracted with DCM (3×20 ml).The combined organic phases were washed with H2O (30 ml) and dried over anhydrous MgSO4. Removal of the solvent under reduced pressure gave the crude product, which was purified by Kugelrohr distillation to give the pure cyclic sulfides 1-4. 4.2.1. Tetrahydrothiopyran (1) Yield 1.60 g (80%) from 1,5-dibromopentane (4.00 g, 17.4 mmol) and sodium sulfide nonahydrate (6.27 g, 26.1 mmol) as a colorless oil (Bp 50-55°C at 15 mmHg; lit. 140-141°Cat RT [40]). 1H-NMR (CDCl3) δ (ppm): 1.60 (m, 2 H), 1.85 (m, 4 H), 2.65 (m, 4 H); 13CNMR (CDCl3) δ (ppm): 26.8, 28.1, 29.4; MS- EI+ m/z (%) 102 ([M]+, 100), 87 (95), 67(60), 46 (75), 39 (80).
References:
Smith, Keith;Williams, Des;El-Hiti, Gamal A. [Journal of Sulfur Chemistry,2019,vol. 40,# 5,p. 529 - 538]
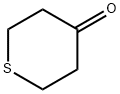
1072-72-6
271 suppliers
$10.00/1g

1613-51-0
90 suppliers
$25.39/1g
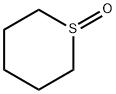
4988-34-5
10 suppliers
inquiry

1613-51-0
90 suppliers
$25.39/1g

1633-79-0
2 suppliers
inquiry

1613-51-0
90 suppliers
$25.39/1g