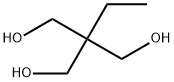
Trimethylolpropane synthesis
- Product Name:Trimethylolpropane
- CAS Number:77-99-6
- Molecular formula:C6H14O3
- Molecular Weight:134.17
922-63-4
95 suppliers
$55.00/1g
50-00-0
838 suppliers
$10.00/25g

77-99-6
509 suppliers
$6.00/100g
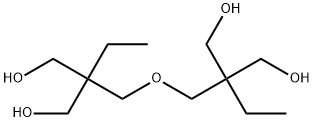
23235-61-2
133 suppliers
$5.00/25g
Yield:77-99-6 253 g ,23235-61-2 66 g
Reaction Conditions:
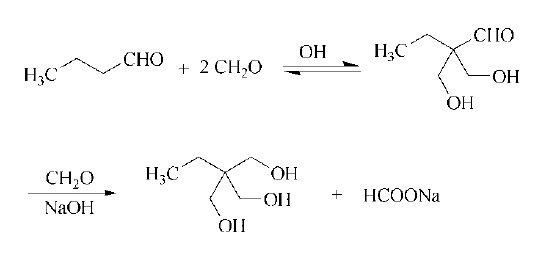
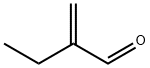
Stage #1: ethylacrolein;formaldehyd in water at 100;
Stage #2: with sodium hydroxide in water at 100;Reagent/catalyst;Time;Concentration;
Steps:
1 Example 1 Addition of 0.81 Mol of TMP in First Step; Split Addition of Formaldehyde; Split Addition of Base
Third Step: The simultaneous dropwise addition of the resulting distillate and 140 g of a 40% by mass formaldehyde (2) aqueous solution (1.9 mol as formaldehyde; 0.99 equivalent based on NBD; 28% of the total used amount of formaldehyde) into the reaction vessel was initiated using two feed pumps, and both were added dropwise to the reaction mixture solution for 19 min and 68 min, respectively, while maintaining the temperature of the reaction mixture solution at 100° C. After completion of the dropwise addition, the resultant was held under heating at 100° C. for 30 min, and then 22.6 g of a 20% by mass sodium hydroxide aqueous solution (0.11 mol as base; 0.06 equivalent based on NBD; 6% of total used amount of base) were added dropwise thereto using a feed pump over 12 min. After completion of the dropwise addition, the resultant was further held under heating at 100° C. for 39 min. [0089] As a result of analyzing the resulting reaction mixture solution by GC, it was confirmed that the amounts of TMP and di-TMP as calculated values were 253 g and 66.0 g, respectively, and the yields were 57.5% and 28.1%, respectively, on the basis of NBD as the raw material and the total yield of TMP and di-TMP was 85.6%. The amount of TMP newly produced by the reaction corresponds to 134% of an amount of TMP required in the subsequent reaction when it was recycled as the raw material in the subsequent reaction. The results are shown in Tables 1 and 2.
References:
US2014/135536,2014,A1 Location in patent:Paragraph 0088-0089

123-72-8
401 suppliers
$12.32/25ML

77-99-6
509 suppliers
$6.00/100g
50-00-0
838 suppliers
$10.00/25g

123-72-8
401 suppliers
$12.32/25ML

77-99-6
509 suppliers
$6.00/100g
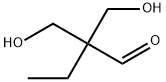
41966-25-0
5 suppliers
inquiry

77-99-6
509 suppliers
$6.00/100g
50-00-0
838 suppliers
$10.00/25g

123-72-8
401 suppliers
$12.32/25ML

67-56-1
726 suppliers
$9.00/25ml

77-99-6
509 suppliers
$6.00/100g