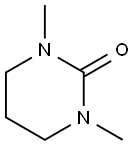
1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone(DMPU): a better space-demanding solvent
Nov 14,2019
1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (CAS no. 7226-23-5), also called N,N'-dimethylpropyleneurea or DMPU, is a polar aprotic solvent with a wide range of uses including solvent for organic reactions and additive in paints and plastics[1]. It is a cyclic urea and used as a polar aprotic organic solvent[2]. It can also be used for surface cleaning and in hydrocarbon extractions, as the hygroscopic liquid is completely miscible in water. Trichloromethane (chloroform) and dichloromethane (methylene chloride), however, do retain DMPU in the organic phase[3].
In 1985, Dieter Seebach showed that it is possible to substitute the relatively toxic hexamethylphosphoramide (HMPA) with DMPU[4]. Later, DMPU was developed as a less harmful substitute for the carcinogenic substances 1,1,3,3-tetramethylurea (TMU) and hexamethyltriphosphoric amide(HMPA), but it is possible that also DMPU may act as a possible chemical mutagen. TMU and HMPA were both used in the many organic and organometallic reactions which needed a dipolar, aprotic co-solvent. Especially HMPA was used extensively, as it is able to strongly solvate cations, but this changed when the carcinogenic activity was noted and the compound was classified as such. Also N,N’-dimethylethyleneurea, DMEU was developed as another safe HMPA substitute, which is effectively used in organic reactions with high basicity[3].
The first recorded use of DMPU as a coordinating ligand was made by Anderson and Smith in 1990, and were followed by a few papers by the Carmalt and Norman research group in the first half of that decade. Pure DMPU was studied crystallographically by Armstrong et al. in 1992. More recent studies include two Japanese research groups, studying lanthanoid catalysts. This thesis and the work surrounding it are part of an extended DMPU study by the Persson group with many publications since the first one appeared in 2000[3].
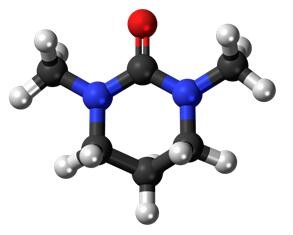
The ability to imitate the unreactive, dipolar, and aprotic properties of HMPA was the original justification for developing DMPU (in addition to being noncarcinogenic). This unreactivity is likely caused by the two methyl groups which border the electron donating oxygen atom and a semi-rigid ring structure. The molecule is said to be space-demanding (at coordination), that is having spatial requirements due to surrounding, non-coordinating atoms (in this case, the methyl groups). These methyl groups have been shown to obstruct the standard coordination chemistry seen in less space-demanding solvents, forming solvates with lower coordination number just like TMU and HMPA. Other properties that make DMPU a good solvating agent are its relatively high electron-pair donor ability, high permittivity, high dipole moment, and a wide liquid range. Solvates incorporating a space-demanding solvent molecule, such as DMPU, may show a decrease in the coordination number, and a lowering of this kind may alter the chemical reactivity significantly. When compared with their six-coordinated counterparts, five-coordinated solvates, such as nickel(II) in DMPU, show a marked higher ability to form complexes. In spite of their carcinogenic properties, both HMPA and TMU have been thoroughly studied over the years. A search in the Cambridge Structural Database (CSD) returns well over 30 structures, both homoleptic and heteroleptic[3].
Reference
[1] Topel, et al. "On the structure of the N,N′-dimethylpropyleneurea and dimethylsulfoxide solvated gallium(III) and indium(III) ions and bromide complexes in solution and solid state, and the complex formation of the gallium(III) and indium(III) bromide systems in N,N′-dimethylpropyleneurea." Inorganica Chimica Acta 363.5(2010):988-994.
[2] https://www.alfa.com/zh-cn/catalog/L09968/
[3] Lundberg, Daniel . "The coordination chemistry of solvated metal ions in DMPU." Doctoral Diss (2006).
[4] https://en.wikipedia.org/wiki/DMPU
- Related articles
- Related Qustion
Berberine hydrochloride is a natural bioactive nitrogen-containing compound that belongs to the protoberberine hydrochloride group of isoquinoline alkaloids.....
Nov 14,2019Drugs1, 6-dibromopyrene is a kind of pyrene chemical. It is majorly used as organic intermediate, OLED (organic light-emitting diodes) materials and photoelectric material.....
Nov 14,2019Organic Synthesis IntermediateYou may like
1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone manufacturers
- 1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone
-

- $1.00 / 500g
- 2025-12-15
- CAS:7226-23-5
- Min. Order: 300g
- Purity: 99.8%
- Supply Ability: 20 TONS
- 1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone
-
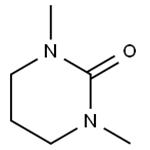
- 2025-12-15
- CAS:7226-23-5
- Min. Order:
- Purity: 0.99
- Supply Ability:
- 1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone
-

- $0.00 / 25KG
- 2025-12-11
- CAS:7226-23-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt