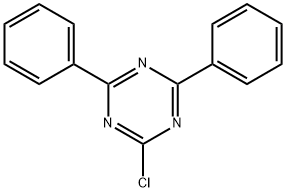
2-chloro-4,6-diphenyl-1,3,5-triazine - reaction / application on synthetic works
Nov 12,2019
2-chloro-4,6-diphenyl-1,3,5-triazine is an important organic intermediate to synthetize novel bipolar host materials based on 1, 3, 5-triazine derivatives for highly efficient phosphorescent OLEDs with extremely low efficiency roll-off, the triazine series including a new synthesis of 1: 2: 4-triazines, and host materials for phosphorescent organic light-emitting diodes by modifying the 1-position of carbazole
The following example is about its application on the synthesis of organic and display device [1]
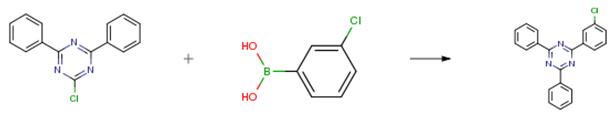
Nitrogen in 2-chloro -4, 6-diphenyl -1, 3, 5-triazine (2-chloro-4, 6-diphenyl-1, 3, 5-triazine) 100 g (374 mmol) senses a rotation velocity of the disk to a for ease of application (THF) 900 ml, phenyl step theory it buys chloro 3-herein (3-chlorophenylboronic acid) 70.1g (448 mmol) and a tetrakis (triphenyl phosphine) palladium (tetrakis (triphenylphosphine) palladium) 4.32 g (3.74 mmol) visitor is checked through a fifth lens. Cyclocarbonate potassium saturated in water (potassium carbonate) 129 g (935 mmol) in 80 °C doesn't have any error frames, the reflux by heating. Complete after water reaction solution, followed by methane doesn't have any error frames, MgSO4 anhydride after removing the water, filter and was concentrating it under reduced pressure. Thus-obtained residue flash column purification product 117 g (91 percent) is obtained.
The following example is about its application on the synthesis of bicarbazole compounds for OLEDS [2]
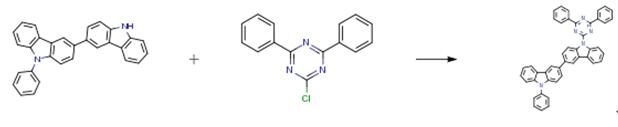
To a solution of sodium hydride (100 mg, 3.0 mmol) and 3-(9- phenyl-9H-carbazol-3- yl)-9H-carbazole (816 mg, 2.0 mmol) in dry DMF (40 mL) was stirred at room temperature for 1 h under argon atmosphere. 2-Chloro-4,6-diphenyl-l,3,5-triazine (448 mg, 1.67 mmol ) was added to the solution at room temperature, then refluxed overnight. The mixture was poured into water and the precipitation was collected by filtration and washed with water, methanol and DCM to get 800 mg (75percent) yellow solid.
The following example is about its application on the synthesis of organic optoelectric device [3]

Nitrogen environment at intermediate (20 g, 39.5 mmol) tetrahydrofuran (THF) and then dissolved in 0.2 L, 4-chloro-2's , 6-diphenyl-1,3,5-triazine (10.6 g, 39.5mmol) and into the tetrakis (triphenylphosphine) palladium (0.46 g, 0.4 mmol) was stirred. Into the potassuim carbonate (13.6 g, 98.8 mmol) in saturated water it was heated to reflux for 23 hours. After the reaction was completed, the reaction solution into water and extracted with dichloromethane (DCM) and then water was removed with anhydrous MgSO4, filter and was concentrated under reduced pressure. Thus, the obtained residue was purified by flash column chromatography purification to obtain the product (17.9 g, 74 percent).
The following example is about its application on the synthesis of display device [4]

In the nitrogen ambient, after the compound 1-120 g (56.5 mmol) was melted in the tetrahydrofuran (THF) 0.2 L here 2- chloro-4,6- diphenyl -1,3,5- triazine (2-chloro -4,6-diphenyl-1,3,5-triazine) 15.1 g (56.5 mmol) and tetrakis (triphenylphosphine) palladium(tetrakis (triphenylphosphine)palladium) 0.65 g (0.57 mmol) were put and it mixed. The saturated potassium carbonate 19.5 g (141 mmol) was put in water and it heated up in 80 for 20 hours and it refluxed. After water was put into the reaction solution after the reaction completion and it extracted in the dichloromethane (DCM) moisture was removed to the anhydrous MgSO4 it filtered and it was concentrated under reduced pressure. The residue obtained in this way was refined to the flash column chromatography after dividing and the product (22.1 g, 85 %) was obtained.
References
1. Cheil Industries Inc. Lee H, Yu D, Yu ES, Hong JS, Kang DM, Shin JH, Jeong SY, Han SJ. Organic compound and organic optoelectric device and display device. KR2015/16845[P], 2015, A. Paragraph 0203-0205.
2. Universal Display Corporation. Xia C, Kwong R, Wong KT, Ming-Cheng K. Bicarbazole compounds for OLEDS. WO2012/23947[P], 2012, A1, Page column 142-143.
3. Samsung SDI Co. Ltd. Lee HI, Ryu DW, Ryu JH, Shin CJ, Yoo ES, Jung SH. Composition for organic optoelectric device and organic optoelectric device and display device. KR2016/11036[P], 2016, A, Paragraph 0363 - 0367.
4. Cheil Industries Co. Ltd. Oh JJ. Kang GW. Kang US, Kim YH, Kim H, Yang YT, Lee HI, Jo PS. Composition for organic optoelectric device and organic optoelectric device and display device. KR2015/28579[P], 2015, A, Paragraph 0309-0312.
- Related articles
- Related Qustion
- 2-Chloro-4,6-diphenyl-1,3,5-triazine: properties, applications and safety Dec 11, 2023
2-Chloro-4,6-diphenyl-1,3,5-triazine is used in organic synthesis and electronics, but its handling requires caution due to safety concerns and potential harm to humans and aquatic life.
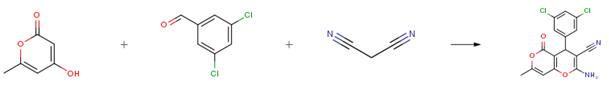
3,5-Dichlorobenzaldehyde is an important organic intermediate to synthetize substituted benzene products.....
Nov 12,2019Aromatic aldehydesTesmilifene is a small molecule chemopotentiator under development by YM BioSciences, a Candian pharmaceutical company that specialises in the development of cancer treatments.....
Nov 12,2019API2-chloro-4,6-diphenyl-1,3,5-triazine
3842-55-5You may like
2-chloro-4,6-diphenyl-1,3,5-triazine manufacturers
- 2-Chloro-4,6-diphenyl-1,3,5-triazine
-

- $0.00 / 25KG
- 2025-04-30
- CAS:3842-55-5
- Min. Order: 1KG
- Purity: ≥99.5%
- Supply Ability: 100mt/year
- 2-Chloro-4,6-diphenyl-1,3,5-triazine
-

- $0.00 / 1KG
- 2025-04-30
- CAS:3842-55-5
- Min. Order: 10g
- Purity: 99.5%
- Supply Ability: 5TONS
- 1,3,5-Triazine, 2-chloro-4,6-diphenyl-
-
- $0.00 / 1KG
- 2025-04-28
- CAS:3842-55-5
- Min. Order: 1KG
- Purity: 98.00%
- Supply Ability: 150KG /month