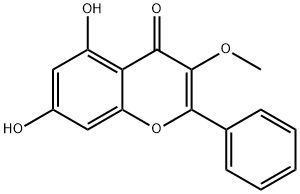
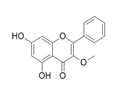
Application of Galangin-3-methylether
Dec 26,2019
Galangin-3-methylether is an important organic building block to synthetize substituted galangin products.
The following example is about its application on the synthesis of flavonoids [1]

Potassium persulfate (9.5 g;35.1 mmol) in 250 mL of water was added to 5 g of starting material, alkene (17.6 mmol) in 50 mL of 20% aq. tetraethylammonium hydroxide. The solution was stirred for 2 h in the dark under nitrogen. After neutralization with a saturated aq solution of KH2PO4, the mixture was extracted with Et2O, followed by butanol. After evaporation of the butanolic extract, the residue wasdissolved in 60 mL of 10% aq. tetramethylammonium hydroxide and, after addition of 6 mL of prenyl bromide (52 mmol), stirred for 1 h. The reaction was stopped by acidification with 6 N HCl. After 4 h at room temperature, the solution was extracted with EtOAc. 2.5 g of the product (6.8 mmol; yield 39%) were obtained after purification of the extract by MPLC on polyamide SC6 (toluene).
The following example is about its application on the synthesis of 7-vinylflavone and 7-aminoflavone [2]

To a solution of 5,7-dihydroxy- to give 5-hydroxy-3-methoxy-7-triflyloxyflavone (1.02 g, 4.0 mmol) in 30 mL of dichloromethane and 10 mL of pyridine at 0°C was slowly added trifluoromethanesul- and 3-methoxy-5,7-bis(triflyloxy)flavone in 1 mL of dichlorometh- heptane/EtOAc. The mixture was allowed to warm to room temperature and then stirred for 22.5 h. Thereafter, it was concentrated and the residue was chromatographed (heptane/EtOAc, 8:1) to give the product as pale-yellow needles (1.13 g, 73%)
The following example is about its application on the synthesis of peracetylated benzopyranones [3]

The peracetylated derivatives were prepared by acetylation of the corresponding polyphenolic compounds by acetic anhydridepyridine method either at room temp. (32 "C) or at 100 "C. The earlier known acetates were identified by comparison of their spectral data with those reported in the literature, while the new acetylated compounds were unambiguously identified on the basis of their 1H NMR and IR spectral data.
References
1.Daskiewicz JB, Depeint F, Viornery L, Bayet C, Comte-Sarrazin G, Comte G, Gee JM, Hostettmann K, Barron D. Effects of flavonoids on cell proliferation and caspase activation in a human colonic cell line HT29: An SAR study[J]. Journal of Medicinal Chemistry, 2005, 48(8):2790-2804.
2.Deng BL, Lepoivre JA, Lemiere G. Synthesis of 7-vinylflavone and 7-aminoflavone by palladium-catalyzed coupling reactions[J]. European Journal of Organic Chemistry, 1999, 10:2683-2688
3.Parmar P, Sharma V, Pati SB. Lipase-catalysed selective deacetylation of peracetylated benzopyranones[J]. Journal of the Chemical Society. Chemical communications, 1993, 1:27-29
- Related articles
- Related Qustion
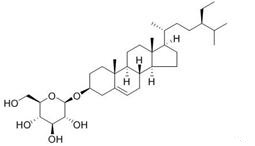
Daucosterol, alias: Citoside, β-Sitosterol β-D-glucoside, and the molecular formula is C35H6. The compound of the formula (description column) belongs to a carotene compound.....
Dec 26,2019Plant extracts3-bromodibenzo[b,d]thiophene is an important organic intermediate to synthetize substituted dibenzo[b,d]thiophene products.....
Dec 26,2019Organic ChemistryGALANGIN-3-METHYLETHER
6665-74-3You may like
- Exploring the Compound H2O: Water
Feb 27, 2024
- Does the Molecule of Water Have Ionic Charge?
Feb 5, 2024
- The uses of 2',7'-Dichlorofluorescein
Jan 18, 2024
GALANGIN-3-METHYLETHER manufacturers
- Galangin 3-methyl ether
-
- $0.00 / 20mg
- 2023-02-24
- CAS:6665-74-3
- Min. Order: 20mg
- Purity: ≥98%(HPLC)
- Supply Ability: 10 g