Articaine hydrochloride--Potency, Toxicity, Metabolism, Excretion, Pediatric use and recommended dose
Oct 13,2020
Pertinent Information
Classification. Hybrid molecule. Classified as an amide; however, it possesses both amide and ester characteristics.
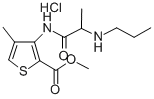
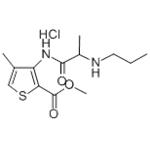
Chemical formula. 3-N-Propylaminoproprionylamino- 2-carbomethoxy-4-methylthiophene hydrochloride. Prepared by. H. Ruschig, G. Ehrhart 1969.
FDA approved. April 2000.
Introduced. 1976 in Germany and Switzerland, 1983 in Canada, 2000 in the United States.
Potency. 1.5 times that of lidocaine; 1.9 times that of procaine. Toxicity. Similar to that of lidocaine and procaine.
Metabolism. Articaine is the only amide local anesthetic containing a thiophene aromatic ring rather than benzene.
Because articaine hydrochloride is the only widely used amide local anesthetic that also contains an ester group, biotransformation of articaine hydrochloride occurs in both plasma (hydrolysis by plasma esterase—similarly to other ester local anesthetics) and liver (hepatic microsomal enzymes—similarly to other amide local anesthetics).
The metabolism of articaine hydrochloride is initiated by hydrolysis of the carboxylic acid ester groups to obtain free carboxylic acid.53 Its primary metabolite, articainic acid, is pharmacologically inactive, undergoing additional biotransformation to form articainic acid glucuronide.53 Additional metabolites have been detected in animal studies.54 From this point the reaction can follow several pathways: cleavage of carboxylic acid, formation of an acid amino group by internal cyclization, and oxidation.
Excretion. Via the kidneys; approximately 5% to 10% unchanged, approximately 90% metabolites (M1 at 87%, M2 at 2%).
Vasodilating properties. Articaine has a vasodilating effect equal to that of lidocaine. Procaine is slightly more vasoactive. pKa. 7.8.
pH of plain solution. Not available in North America (4% “plain” articaine hydrochloride is available in Germany). pH of vasoconstrictor-containing solution. 4.0 to 5.5. Onset of action. The onset of anesthesia has been shown to be within 1 to 9 minutes of injection of articaine. Pulpal anesthesia lasts approximately 1 hour for infiltrations and up to approximately 2 hours following nerve block.
Effective dental concentration. 4% with epinephrine 1:100,000 or 1:200,000. Articaine hydrochloride is available with epinephrine 1:400,000 in Germany.
Anesthetic half-life. 0.5 hours (27 minutes). Topical anesthetic action. Not in clinically acceptable concentrations. Pregnancy classification. C.
Nursing mothers. It is not known whether articaine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when articaine is administered to a nursing woman. When using articaine, nursing mothers may choose to pump and discard breast milk for approximately 4 hours (based on plasma half-life) following an injection of articaine (to minimize infant ingestion) and then resume breastfeeding. Pediatric use. Safety and efficacy of articaine in pediatric patients below the age of younger than 4 years have not been established. The quantity of articaine hydrochloride in children aged 4 to 16 years to be injected should be determined by the age and weight of the child and the magnitude of the operation. The maximum dose of 4% articaine hydrochloride should not exceed 7 mg/kg.
Maximum recommended dose. The FDA MRD is 7.0 mg/ kg body weight, or 3.2 mg/lb body weight, for the adult patient.
Comments. Originally known as carticaine, the generic nomenclature of this local anesthetic was changed in 1984 to articaine. Literature appearing before 1984 should be reviewed under the original name.
Articaine is the only amide anesthetic to possess a thiophene ring as its lipophilic moiety. It has many of the physicochemical properties of other amide and ester local anesthetics, with the exception of the aromatic moiety and the degree of protein binding.
Articaine has been available in Europe since 1976 and in Canada since 1983 in two formulations: 4% with epinephrine 1:100,000 and 4% with epinephrine 1:200,000 (Fig. 4.5). In May 2000 the FDA approved articaine hydrochloride with epinephrine 1:100,000 for marketing in the United States.58-60 The formulation with epinephrine 1:100:000 provides between 60 and 75 minutes of pulpal anesthesia; the 1:200,000 formulation provides approximately 45 to 60 minutes.
Since its introduction into the US dental market in May 2000, articaine has steadily become increasingly popular. In 2018 articaine was the second most used dental local anesthetic in the United States (∼39.3% market share).63 Articaine, as the most recently marketed dental local anesthetic, has been the subject of intense discussion and of many (anecdotal) claims made by dentists, some good (faster onset, increased success rates; “don’t miss as often”), some bad (increased risk of paresthesia). It has been claimed that articaine is able to diffuse through soft and hard tissues more reliably than other local anesthetics. Clinically, it is claimed that following maxillary buccal infiltration, articaine on occasion may provide palatal soft tissue anesthesia, obviating the need for palatal injection, which, in many hands, is traumatic.
Some initial claims about articaine have been shown to be true, specifically, the significant success of articaine administered by buccal infiltration in the mandible of adult patients. The success of articaine in the United States mirrors its success elsewhere. In Germany, the first country to have articaine (1976), by 1989 it was used by 71.7% of German dentists, and by 2010 it commanded 95% of the German dental local anesthetic market. Articaine has become the leading local anesthetic in Canada, which acquired it in 1983. In the United States, where articaine has been available since June 2000, it commanded 38.4% of the local anesthetic market in 2014, increasing to 39.3% in 2018.
World wide, articaine is the second most used dental local anesthetic with approximately 600, 000, 000 cartridges manufactured annually.
Reports of paresthesia following local anesthetic administration became more frequent after the introduction of articaine into the United States. An overwhelming majority of reported cases occurred following inferior alveolar nerve block and primarily involved the lingual nerve.
Articaine, like other local anesthetics, can cause methemoglobinemia, particularly in conjunction with methemoglobin- inducing agents. Therefore articaine is relatively contraindicated in patients with congenital or idiopathic methemoglobinemia, or in patients who are receiving treatment with methemoglobin-inducing agents because they are more susceptible to drug-induced methemoglobinemia.
Such reactions had been noted after the intravenous administration of articaine for regional anesthetic purposes; however, no cases have been reported when articaine was administered in the usual manner and volume for dental procedures.
Articaine hydrochloride with epinephrine is contraindicated in persons with known sensitivity to amide-type local anesthetics (few to none) and in persons with sulfite sensitivity (such as some asthmatic patients with allergic-type asthma, as epinephrine-containing local anesthetic formulations contain the antioxidant sodium metabisulfite). Articaine hydrochloride should be used with caution in persons with hepatic disease and significant impairment of cardiovascular function because amide-type local anesthetics undergo biotransformation in the liver and have myocardial depressant properties. Articaine is listed by the FDA as a class C drug during pregnancy. It should be used with caution in women who are nursing because it is not known whether articaine is excreted in milk.55,56 When articaine is administered to a nursing woman, the FDA recommends a 4-hour period of “pump and discard” to ensure that the infant does not receive the drug through breast milk.
Administration to children younger than 4 years is not recommended because insufficient data are available to support such use. Cartridges of articaine marketed in the United States are listed as containing “1.7 mL” , in contrast to other local anesthetic cartridges, which are labeled as containing “1.8 mL.” Some might interpret this to mean that the cartridge consists of 68 mg. This is incorrect. Articaine hydrochloride cartridges are identical in all ways to other dental cartridges. However, as discussed earlier in this chapter, a change was made to labeling, not to the content of the cartridge.
- Related articles
- Related Qustion
- Articaine hydrochloride: pharmacology, efficacy and side effects Nov 23, 2023
Articaine hydrochloride is a local anesthetic with fast onset, long duration, and effective pain relief, but caution should be exercised due to potential side effects.
- What is Articaine hydrochloride? Sep 8, 2021
Articaine is a dental amide-type local anesthetic. It is the most widely used local anesthetic in a number of European countries and is available in many countries.
Prilocaine hydrochloride (Citanest) is an amide anesthetic whose onset of action is slightly longer than that of lidocaine; its duration of action is comparable.....
Oct 12,2020AnestheticsBupivacaine hydrochloride has particularly long action, and some nerve blocks last more than 24 hours; this is often an advantage for postoperative analgesia.....
Oct 15,2020AnestheticsArticaine hydrochloride
23964-57-0You may like
- Lidocaine hydrochloride: A local anesthetic
Mar 19, 2025
- What is Atracurium Besylate Injection
Apr 15, 2024
- General Introduction of Caffeine
Mar 3, 2022
Articaine hydrochloride manufacturers
- Articaine hydrochloride
-
- 2025-07-16
- CAS:23964-57-0
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Articaine hydrochloride
-

- $0.00 / 1kg
- 2025-07-16
- CAS:23964-57-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000
- Articaine hydrochloride
-

- $0.00 / 1kg
- 2025-07-16
- CAS:23964-57-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 500