Learn more about Glyoxylic Acid
Sep 16,2019
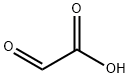
Glyoxylic acid or oxoacetic acid is an organic compound that is both an aldehyde and a carboxylic acid. Glyoxylic acid is a liquid with a melting point of -93°C and a boiling point of 111°C. It is an intermediate of the glyoxylate cycle, which enables certain organisms to convert fatty acids into carbohydrates.
In humans, glyoxylate is produced via two pathways: (1) through the oxidation of glycolate in peroxisomes and (2) through the catabolism of hydroxyproline in mitochondria. In the peroxisomes, glyoxylate is converted into glycine by glyoxylate aminotransferase (AGT1) or into oxalate by glycolate oxidase. In the mitochondria, glyoxylate is converted into glycine by mitochondrial glyoxylate aminotransferase AGT2 or into glycolate by glycolate reductase. A small amount of glyoxylate is converted into oxalate by cytoplasmic lactate dehydrogenase. Glyoxylic acid is found to be associated with primary hyperoxaluria I, which is an inborn error of metabolism. Under certain circumstances, glyoxylate can be a nephrotoxin and a metabotoxin. A nephrotoxin is a compound that causes damage to the kidney and kidney tissues. A metabotoxin is an endogenously produced metabolite that causes adverse health effects at chronically high levels.
High levels of glyoxylate are involved in the development of hyperoxaluria, a key cause of nephrolithiasis (commonly known as kidney stones). Glyoxylate is both a substrate and inductor of sulfate anion transporter-1 (SAT-1), a gene responsible for oxalate transportation, allowing it to increase SAT-1 mRNA expression, and as a result oxalate efflux from the cell. The increased oxalate release allows the buildup of calcium oxalate in the urine, and thus the eventual formation of kidney stones. As an aldehyde, glyoxylate is also highly reactive and will modify proteins to form advanced glycation products (AGEs).
Aldehyde Acids and Keto Acids
Aldehyde acids and keto acids include compounds such as glyoxylic acid (oxoacetic acid), pyruvic acid, and levulinic acid (4-oxopentanoic acid). Similar to the situation of other compounds with more functional groups, the second group can be in the α, β, γ, etc., position to the carboxyl. Aldehyde and keto acids also include more complex compounds, with some resulting from the oxidation of sugars. The sugar acids from this group contain the OH functionality besides carboxyl and carbonyl, and include uronic acids and ketoaldonic acids. Pyrolysis of a uronic acid (glucuronic acid) was described in Subchapter 11.3.
Glyoxylic acid is the simplest α-carbonyl organic acid. At temperatures above 100°C, thermal decomposition of glyoxylic acid monohydrate in a sealed tube generates oxalic acid and glycolic acid, due to a Cannizzaro-type reaction of the aldehyde group (see reaction 2.5.1). During thermal decomposition in a static system in the temperature range 200–537°C and pressures between 0.4 and 8 Torr, the main pyrolysis products were CO2 and HCHO [3].

The first-order rate constant for the formation of CO2 in reaction (12.4.7) is given by the expression:
(12.4.8)log10ks−1=7.80−30.8kcal/mol/2.303RT
Besides carbon dioxide and formaldehyde, low levels of H2, CO, and H2O (CO less than 10% of CO2 level) also are formed during glyoxylic acid thermal decomposition, and the ratio CO/CO2 increases as the temperature increases. The levels of HCHO in the pyrolyzate were somewhat lower than those of CO2, which indicates either some decomposition of HCHO formed in reaction (12.4.7) or the formation of CO2 by other mechanisms. The decomposition of glyoxylic acid induced by a pulsed laser with estimated temperatures between 1100 K and 1600 K showed the same behavior as the static system [3]. It is likely that the formation of CO and H2 is a result of CO elimination with HCOOH formation, with the formic acid being further decomposed into CO and H2O.
Pyruvic acid (2-oxopropanoic acid) is a α-keto acid, which can be considered as derived from glyoxylic acid with a CH3 substituent to the hydrogen atom bound to the CO group. During pyrolysis, this compound generates mainly CO2 and acetaldehyde. The formation of acetic acid and CO also takes place, but to a lower extent. The two reactions are shown below:
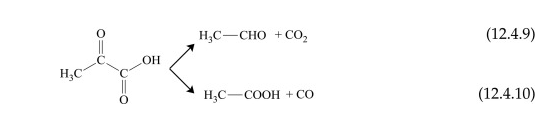
Pyrolysis of pyruvic acid also leads to the formation of some condensation products.
Similar to pyruvic acid, phenylglyoxylic acid (phenyl substituted to glyoxylic acid)
decomposes above 300°C with the formation of CO2, CO, benzaldehyde, and benzoic acid.
Particular substituents to glyoxylic acid may influence the pyrolysis outcome. A thienyl group, for example, leads to the generation of CO2 exclusively and the formation of thiophene-2-caboxaldehyde (in addition to some charring).
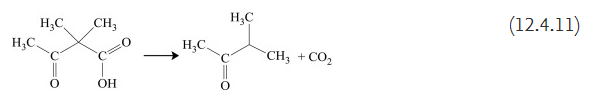
Pyrolysis of β-ketonic acids typically leads to the elimination of CO2 and the formation of a ketone. Ketones are in general relatively stable to heating, and if a carboxyl group is attached to the same molecule, the decarboxylation process usually takes place before the ketone decomposition. Acetoacetic acid (3-oxobutanoic acid), for example, is not very stable and decomposes into CO2 and acetone below 100°C. Similarly, 2,2-dimethyl-3-oxobutanoic acid generates CO2 and methylisopropyl ketone.
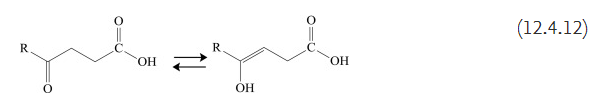
γ-Ketonic acids and δ-ketonic acids behave differently from α and β-ketonic acids. The keto-enol equilibrium of these compounds allows them to exist (in part) in two forms, as shown below:
The enol form has the capability to generate lactones by water elimination in the first stages of pyrolysis. The process can be written as follows:
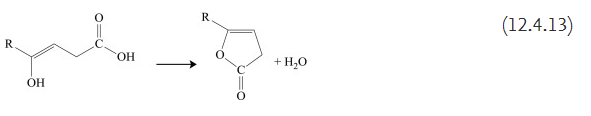
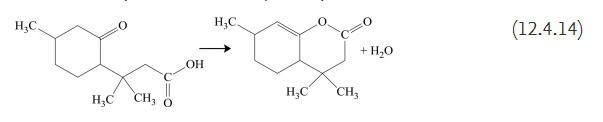
Formation of furanones is characteristic for levulinic acid (4-oxopentanoic acid) as well as for several other similar acids such as 2-methyl-4-oxopentanoic acid, 2-ethyl-4-oxo-pentanoic acid, and 3-methyl-4-oxopentanoic acid. In reactions similar to (12.4.13), δ-ketonic acids generate pyranones. Cyclic lactones are formed even when the carbonyl group is part of a cycloalkane, as shown below for 3-methyl-3-(4-methyl-2-oxo-cyclohexyl)butanoic acid that generates 4,4,7-trimethyl-3H-4,5,6,7,4a-pentahydrochromen-2-one:
If the formation from the ketone of an enol is not possible (e.g., a double bond exists in the β-position to the carbonyl), the decomposition of the keto acids takes place with simple decarboxylation. Some substituents such as CN positioned between the CO group and the COOH group were found to influence the pyrolysis results and favor decarboxylation [4].
Ketodicarboxylic acids behave similarly to monocarboxylic keto acids. For example, o-carboxyphenylglyoxylic acid or 2-(carboxycarbonyl)benzoic acid by thermal decomposition around 180°C generates both o-carboxybenzaldehyde by CO2 elimination and phthalic anhydride by elimination of CO and then of H2O from the intermediate phthalic acid.
- Related articles
- Related Qustion
- What are the roles of glyoxylic acid and glycolic acid in hair? Jun 24, 2024
Glyoxylic acid (GA) is a straightening agent for hair care products. However, due to the continued use of Glyoxylic acid products can produce odour and discolouration.
- What is glyoxylic acid used for hair? Apr 19, 2024
Glyoxylic acid (GA) is widely used as a straight perming agent for hair care products; it penetrates deeply to repair, nourish, and reshape the hair shaft, allowing an intense and long-lasting smoothing action.
- Glyoxylic acid: Application, Production and Isolation May 10, 2023
Glyoxylic acid or oxoacetic acid is an organic compound that is both an aldehyde and a carboxylic acid.
Dicyclohexylamine is a secondary amine with the chemical formula HN(C6H11)2. It is a colorless liquid, although commercial samples can appear yellow. It has a fishy odor, typical for amines. It is sparingly soluble in water. As an amine, it....
Sep 16,2019Organic ChemistryAlso known as methylene chloride, dichloromethane (DCM) is a transparent, colorless, volatile halogenated aliphatic hydrocarbon compound with an ether-like mildly sweet smell. It is moderately soluble in water as well as in most organic sol....
Sep 16,2019Organic ChemistryGlyoxylic acid
298-12-4You may like
- Glyoxylic acid
-

- $0.00 / 1KG
- 2025-06-16
- CAS:298-12-4
- Min. Order: 1KG
- Purity: 50.30%
- Supply Ability: 50tons/month
- Glyoxylic Acid monohydrate
-

- $0.00 / 1Kg/Bag
- 2025-06-16
- CAS:298-12-4
- Min. Order: 1Kg/Bag
- Purity: 99% up, High Density
- Supply Ability: 20 tons
- Glyoxylic acid
-

- $0.00 / 200Kg/Drum
- 2025-06-16
- CAS:298-12-4
- Min. Order: 1KG
- Purity: 995
- Supply Ability: 8000mt/year