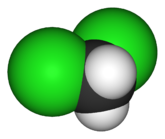
Dichloromethane-Health Hazard and Toxicity
Sep 16,2019
Description
Also known as methylene chloride, dichloromethane (DCM) is a transparent, colorless, volatile halogenated aliphatic hydrocarbon compound with an ether-like mildly sweet smell. It is moderately soluble in water as well as in most organic solvents namely; ether, ethanol, ketones, aldehydes, and phenols.Notably, DCM vapors are heavier than air and are normally non-explosive, stable, and non-flammable when exposed in the air; however, temperatures above 100oC must be avoided. Although natural sources do not largely contribute to the global release of DCM, the latter may lead to the formation of the former.
Toxicity Data
LD50 oral (rat) 1600 mg/kg
LC50 inhal (rat) 88,000 mg/m3; 30 min
PEL (OSHA) 500 ppm (8 h)
TLV-TWA (ACGIH) 50 ppm
Health Hazard
Exposures to methylene chloride cause adverse health effects and poisoning to users. Methylene chloride harms the human CNS. The symptoms of poisoning include, but are not limited to, dizziness, nausea, tingling, and numbness in the fi ngers and toes. Laboratory animals exposed to very high levels of methylene chloride suffer unconsciousness and fatal injury/death. Occupational workers who are exposed to direct skin contact with methylene chloride indicate symptoms of intense burning and mild redness of the skin, damage to the eyes and cornea.
Toxicity
Dichloromethane is classified as only slightly toxic by the oral and inhalation routes. Exposure to high concentrations of dichloromethane vapor (>500 ppm for 8 h) can lead to lightheadedness, fatigue, weakness, and nausea. Contact of the compound with the eyes causes painful irritation and can lead to conjunctivitis and corneal injury if not promptly removed by washing. Dichloromethane is a mild skin irritant, and upon prolonged contact (e.g., under the cover of clothing or shoes) can cause burns after 30 to 60 min exposure. Dichloromethane is not teratogenic at levels up to 4500 ppm or embryotoxic in rats and mice at levels up to 1250 ppm.
Flammability and Explosibility
Noncombustible. Dichloromethane vapor concentrated in a confined or poorly ventilated area can be ignited with a high-energy spark, flame, or high-intensity heat source.
Reactivity and Incompatibility
Reacts violently with alkali metals, aluminum, magnesium powder, potassium tbutoxide, nitrogen tetroxide, and strong oxidizing agents.
Storage and Handling
This compound should be handled in the laboratory using the "basic prudent practices".
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If dichloromethane is ingested, obtain medical attention immediately. If large amounts of this compound are inhaled, move the person to fresh air and seek medical attention at once. In the event of a spill, soak up dichloromethane with a spill pillow or absorbent material, place in an appropriate container, and dispose of properly. Respiratory protection may be necessary in the event of a large spill or release in a confined area.
Disposal
Excess dichloromethane and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
- Related articles
- Related Qustion
- Is CH2Cl2 Polar or Nonpolar? Dec 20, 2023
An electronegativity difference of 0.61 units exists between a carbon and a chlorine atom in the C-Cl bond,makes dichloromethane a polar molecule.
- Toxicity of Dichloromethane Jan 18, 2022
Dichloromethane (DCM; mol. wt. 93.328) was first prepared in 1840 by mixing chloromethane and chlorine and exposed to sunshine. It has been used as a versatile solvent to dissolve various organic compounds in many chemical processes since W
- Toxicity hazards of Dichloromethane Nov 18, 2021
Dichloromethane can be used as a solvent, dental local anesthetic, refrigerant and fire extinguishing agent. In addition to being used in organic synthesis, dichloromethane is often used as a non-flammable solvent for flammable materials.
Glyoxylic acid or oxoacetic acid is an organic compound that is both an aldehyde and a carboxylic acid. Glyoxylic acid is a liquid with a melting point of -93°C and a boiling point of 111°C. It is an intermediate of the glyoxylate cycle, wh....
Sep 16,2019Chemical ReagentsN-Nitrosodiethylamine (NDEA) is a carcinogenic and mutagenic organic compound, classified as a nitrosamine. It is found in tobacco smoke.N-Nitrosodiethylamine is a constituent of tobacco smoke.....
Sep 16,2019Chemical ReagentsDichloromethane
75-09-2You may like
- DCM
-

- $10.00 / 1KG
- 2025-12-11
- CAS:75-09-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100 mt
- Dichloromethane
-
- $0.00 / 200kg
- 2025-11-19
- CAS:75-09-2
- Min. Order: 20kg
- Purity: 99.0%
- Supply Ability: 20 tons
- Dichloromethane
-

- $450.00 / 10ton
- 2025-09-26
- CAS:75-09-2
- Min. Order: 50ton
- Purity: 99
- Supply Ability: 500 ton