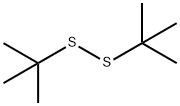
Synthesis and Application of Di-tert-butyl disulfide
Dec 9,2019
Di-tert-butyl disulfide is one of the important organic intermediates.It is an important raw material for the synthesis of disulfide bond-containing sulfinyl and oxythio compounds. It can be used as a vulcanizing agent for rubber and elastomers in industry. It is also a monomer for the synthesis of proteins and other biologically active molecules[1].
The research progress on the synthesis of disulfide compounds is quite rapid, and the synthesis technology is also continuously improved. From ordinary conventional reactions to today's synthesis using many new technologies, such as synthesis under microwave and various phase transfer catalysts.
At present, the methods for synthesizing tert-butyl disulfide in literature reports are mostly based on mercaptans. Due to the availability of mercaptans, the method for preparing disulfide by oxidation with mercaptans has become a common method for the preparation of disulfide. The oxidants used are cerium salts, permanganic acid, perborate, ferric chloride, Chlorite[2], etc., but when these catalysts are used to synthesize tert-butyl disulfide, tertiary butyl mercaptan is a quaternary carbon atom, and the steric hindrance is large, so the method has long reaction time, complicated operation and yield[3]. Low defects. In addition, mercaptans, especially low-carbon chain mercaptans, have a foul smell, are volatile, have serious environmental pollution, and are highly corrosive to equipment[4].

In 2002, Mohammed reported the synthesis of di-tert-butyl disulfide by elemental bromide oxidation. Later, the researchers improved the synthesis of di-tert-butyl disulfide by elementary bromide oxidation. Bromine is an oxidant, Silica gel is used as an endothermic agent and acid binding agent, and zinc powder is used as an oxidation inhibitor, and the product di-tert-butyl disulfide is obtained by oxidation at room temperature[5].

Hu uses sublimed sulfur, sodium hydroxide, t-butyl chloride as raw materials, PEG2400 as a phase transfer catalyst, and phase transfer catalytic synthesis under microwave radiation[6]. This process has the characteristics of easy availability of raw materials, mild reaction conditions, simple operation and high conversion rate. The synthetic route is as follows.

Li uses DMSO complexed MoOCl3 (DMSO) 2 as a catalyst, thiol as a raw material[7], and DMSO as an oxidant for catalytic oxidation, and a series of di-t-butyl disulfide compounds are prepared in high yield.
References
[1] Derbesy, Gerard, Harpp, David N. Detection and Decomposition of Di-tert-butyl Disulfide-Polyoxide Derivatives[J]. Journal of Organic Chemistry, 60(4):1044-1052.
[2] Wolfram Calvet, Carsten Lehmann, Tilo Plake. Epitaxial CuInS2 on Si(111) using di-tert-butyl disulfide as sulphur precursor[J]. Thin Solid Films, 480-481(none):347-351.
[3] D. ELOTHMANI, Q. T. DO, J. SIMONET. ChemInform Abstract: Anodic Oxidation of Di-tert-butyl Disulfide: A Facile Method for the Preparation of N-tert-Butylamides[J]. Cheminform, 2010, 24(35).
[4] Suzanne A Blum, Robert G Bergman, Jonathan A Ellman. Enantioselective Oxidation of Di- tert -Butyl Disulfide with a Vanadium Catalyst: Progress toward Mechanism Elucidation †[J]. Journal of Organic Chemistry, 2003, 68(1):150-155.
[5] Abdul Muttaleb Yousef Jaber, Nemr Ahmed Mehanna, Abdalla Mahmoud Abulkibash. Simultaneous liquid-liquid extraction of dibenzyl disulfide, 2,6-di-tert-butyl-p-cresol, and 1,2,3-benzotriazole from power transformer oil prior to GC and HPLC determination[J]. Journal of Separation Science, 2012, 35(5-6):750-757.
- Related articles
- Related Qustion
4-Formylbenzoic acid is also called 4-Carboxybenzaldehyde. Its molecular formula is C8H5O3, it is insoluble in water, it can be dissolved in DMF, it can undergo esterification reaction and alcohol-aldehyde condensation reaction with alcohol....
Dec 9,2019Organic reagentsL-Tyrosine is an aromatic amino acid and one of the 20 amino acids that synthesize proteins. Tyrosine can be synthesized in the body, so it is a non-essential amino acid.....
Dec 9,2019Amino Acids and DerivativesDi-tert-butyl disulfide
110-06-5You may like
Di-tert-butyl disulfide manufacturers
- DI-TERT-BUTYL DISULFIDE
-

- $1.00 / 1KG
- 2019-12-24
- CAS:110-06-5
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: G/KG/T