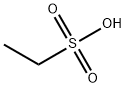
The preparation of ethanesulfonic acid
Dec 20,2019
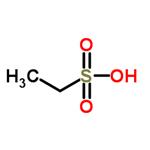
Ethanesulfonic acid is an alkanesulfonic acid in which the alkyl group directly linked to the sulfo functionality is ethyl [1], and it is a conjugate acid of an ethanesulfonate. employed in the electrolytic reduction of perrhenate solutions. Ethanesulfonic acid is used as pharmaceutical intermediates, catalyst of polymerization and alkylation. It participates in the electrolytic reduction of perrhenate solutions.
Let see how to synthesis ethanesulfonic acid. In 1933, a class synthesis route for alkanesulfonic acid preparation starting from ethyl iodide as shown in scheme. The synthesis process was described as below: 20 g of ethyl iodide is boiled under reflux with a solution of 20 g of crystallised ammonium sulfite in 40 ml of water until all goes into solution (6 hours). 100 m of water is added, and the solution boiled with 30 g of lead oxide until all ammonia is expelled. The lead salt of ethylsulfonic acid and lead iodide are formed; the latter is removed by filtration after the solution cools. Hydrogen sulfide is passed into the filtrate until no more lead sulfide from the decomposition of the lead salt of ethylsulfonic acid is formed. Lead sulfide is filtered off, and the filtrate neutralised by the addition of excess (20 g) of barium carbonate. After filtration, the filtrate containing barium ethylsulfonate is evaporated. Final yield is about 90% [2].

Scheme 1 the synthesis of ethanesulfonic acid
Ge Yu and Li Yanfeng developed a synthesis route of alkanesulfonic acid during prepared vascular endothelial growth factor inhibitor compound. The preparation step is just one step starting with 2-oxosuccinic acid as shown in scheme 2 [3]. In the synthesis, fuming nitric acid and sulfuric acid were used and reacted with 2-oxosuccinic acid transferred by decarboxylation of aliphatic acids at 0℃ and get ethanesulfonic acid.
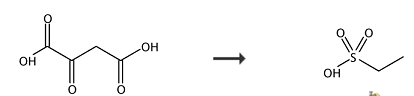
Scheme 2 the synthesis of ethanesulfonic acid
Japanese scientists synthesized ethanesulfonic acid one step with high yield [4]. The typical procedure was described as follows.
(1) To a glass reactor equipped with a reflux condenser, sample inlet, internal cooler, stirring device and solvent was distilled off with an opening and closing cock was charged with 60weight % of hydrogen peroxide solution 295 g (5.20 mol) prepared and 154 g (1.00 mol) of bis(2-hydroxyethyl)disulfide was uniformly added from the sample inlet over 150 minutes and the liquid was stirred. During this time,
the temperature of reaction liquid was held at 45 °C by flowing 19 °C cooling water at the flow rate of 140mL/minutes into the condenser. After completion of the addition, the reaction solution temperature was maintained at 50 °C for 2 hours and heated under reflux for 4 hours.

During heating under reflux, the nitrogen gas was blown and the portion of steam was evaporated from the reaction system. 55 weight% aqueous solution of isethionic acid was obtained as a product. Purity of the generated isethionic acid was 244g (1.94 mol) and the yield was 97%. (2) Similarly, there is another synthesis route as shown in scheme 3. The product was obtained in the same manner as above route (1), instead of bis(2-hydroxyethyl)disulfide 154 g (1.00 mol), diethyl disulfide 122 g(1.00 mol) was added, the temperature was maintained at 45 °C by flowing 19 °C cooling water at the flow rate of 140mL/minute into the condenser and the temperature was maintained at 75 °C for 2 hours. 52 weight% aqueous solution of ethanesulfonic acid was obtained as a product. The purity of the generated ethanesulfonic acid was 213 g (1.94 mol) and the yield was 97%.
reference
[1] https://en.wikipedia.org/wiki/Ethanesulfonic_acid .
[2] https://www.prepchem.com/synthesis-of-ethanesulfonic-acid/
[3] Ge, Yu; Li, Yanfeng, Vascular endothelial growth factor inhibitor compound, CN 106892911
[4] Kobayashi, Hiromitsu and Nito, Hirohisa, Preparation of alkanesulfonic acids from dialkyl disulfides, JP 200415571
- Related articles
- Related Qustion
1H,1H,2H,2H-Perfluorodecyltrichlorosilane, also known as FDTS, is a colorless liquid chemical with molecular formula C10H4Cl3F17Si.....
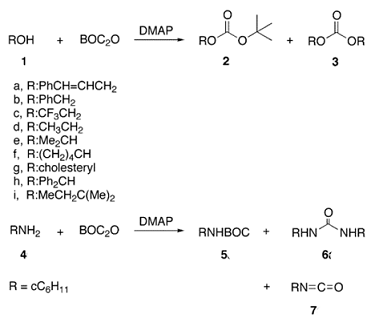
Dec 20,2019Chemical ReagentsDi-tert-butyl dicarbonate ( Boc2O) is mainly used to introduce a tert-butoxycarbonyl (Boc) protecting group to protect an amino group (especially an amino group of an amino acid), and is one of the commonly used reagents for organic synthes....
Dec 20,2019Biochemical EngineeringEthanesulfonic acid
594-45-6You may like
Ethanesulfonic acid manufacturers
- Ethanesulphonic Acid
-

- $100.00 / 25kg
- 2025-07-07
- CAS:94-45-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000KG/month
- Ethanesulfonic acid
-
- $8.00 / 1KG
- 2024-01-22
- CAS:594-45-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Ethanesulfonic acid
-

- $300.00 / 1KG
- 2023-06-26
- CAS:594-45-6
- Min. Order: 10g
- Purity: 99%
- Supply Ability: 500kg/month