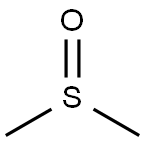
Toxicity of Dimethyl sulfoxide
Jan 19,2022
First synthesized in 1866 by Alexander Zaytsev in the Russian Empire, dimethyl sulfoxide is an organosulfur compound. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water.

Uses
Dimethyl sulfoxide(DMSO) has excellent solvent properties and acts as a skin penetration enhancer for drugs and other substances by increasing the permeability of the barrier layer of the skin. It is used in the topical administration of drugs, the production of synthetic fibers, the application of pesticides, as an antifreeze, hydraulic fluid, and in the manufacturing of industrial cleaners and paint strippers. Its anti-inflammatory and analgesic effects, and the ability to quench free radicals have been used by physicians and others for various therapeutic purposes.
Environmental Fate
DMSO is naturally released in the environment, primarily by
the oxidation of dimethyl sulfide that is biologically produced
in soil, water, and vegetation. It is produced by phytoplankton,
and may be released during its production, transport, disposal,
and use as a solvent, medicinal analgesic, and other uses.
If released in water, it should disproportionate to dimethyl
sulfide and dimethyl sulfone, and may be reduced by reducing
agents that may occur in natural waters. In soil, DMSO is
rapidly reduced to dimethyl sulfide. In the atmosphere, DMSO
will exist primarily in the vapor phase, and will react with
photochemically produced hydroxyl radicals with a half-life of
w7 h. It also may be released during its production, transport,
disposal, and use as a solvent and medical analgesic.
A bioconcentration factor (BCF) of <1 was observed for dimethyl sulfoxide, using orange-red killifish (Oryzias latipes) which were exposed over an 8-week period. According to a classification scheme, this BCF suggests that bioconcentration in aquatic organisms is low.
Physiological properties
Most physiological properties of DMSO appear to be related to
its penetration properties, its potential to inhibit or stimulate
enzymes and to act as a free radical scavenger, and its ability to cause histamine release from mast cells. These properties are
largely based on DMSO’s chemical characteristics, including its
hydrogen bonding behavior, water affinity, ability to interchange
with water in membranes, and ability to react with
organic molecules.
Toxicity
DMSO is a non-toxic solvent with a median lethal dose higher than ethanol (DMSO: LD50, oral, rat, 14,500 mg/kg;ethanol: LD50, oral, rat, 7,060 mg/kg).
Early clinical trials with DMSO were stopped because of questions about its safety, especially its ability to harm the eye. The most commonly reported side effects include headaches and burning and itching on contact with the skin. Strong allergic reactions have been reported.[full citation needed] DMSO can cause contaminants, toxins, and medicines to be absorbed through the skin, which may cause unexpected effects. DMSO is thought to increase the effects of blood thinners, steroids, heart medicines, sedatives, and other drugs. In some cases this could be harmful or dangerous.
- Related articles
- Related Qustion
- Applications of Dimethyl sulfoxide in chemical and medical industry Dec 16, 2024
Dimethyl sulfoxide (DMSO) is a colourless, widely used and powerful non-protonic solvent. It is known as a universal solvent and can be miscible with water and a wide range of organic solvents.
- Dimethyl Sulfoxide: A Widely Used Polar Aprotic Solvent Dec 27, 2023
Dimethyl Sulfoxide (DMSO) is a polar aprotic molecule that has the peculiar property of having a mildly nucleophilic sulfur and a basic oxygen. It is used as a reagent in a variety of different reactions.
- Is Dimethyl sulfoxideharmful to Humans? Dec 1, 2022
DMSO can cause contaminants, toxins, and medicines to be absorbed through the skin, which may cause unexpected effects.
Dimethyl ether, also known as methoxymethane, wood ether, dimethyl oxide or methyl ether, is the simplest ether. It is a colourless, slightly narcotic, non-toxic, highly flammable gas at ambient conditions, but can be handled as a liquid wh....
Jan 19,2022Organic ChemistryThe first indication of the extreme toxicity of dimethylmercury (DMM) was documented in 1863 when two laboratory assistants died of DMM poisoning while synthesizing DMM in the laboratory of Frankland and Duppa. There are numerous reports of....
Jan 19,2022Chemical ReagentsDimethyl sulfoxide
67-68-5You may like
Dimethyl sulfoxide manufacturers
- Dimethyl sulphoxide
-
- 2025-08-03
- CAS:67-68-5
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Dimethyl sulfoxide
-

- $0.00 / 1KG
- 2025-08-01
- CAS:67-68-5
- Min. Order: 1KG
- Purity: >99% GC
- Supply Ability: 1000KG
- Dimethyl sulfoxide
-

- $0.00 / 25KG
- 2025-08-01
- CAS:67-68-5
- Min. Order: 25KG
- Purity: 98%min
- Supply Ability: 30tons/month