General Description
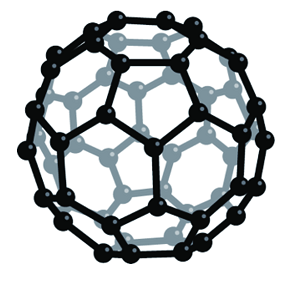
Fullerene C60 is molecular carbon in the form of C60 and other members of the fullerene family were first synthesized in 1985. An allotrope of carbon containing clusters of 60 carbon atoms bound in a highly symmetric polyhedral structure. The C60 polyhedron has a combination of pentagonal and hexagonal faces similar to the panels on a soccer ball. The molecule was named for the American architect Richard Buckminster Fuller (1895–1983) because its structure resembles a geodesic dome (invented by Fuller).
Fullerenes are molecules that composed entirely of carbon. They are similar in structure to graphite, which is composed of a sheet of linked hexagonal and pentagonal rings that prevent the sheet from being planar. Each carbon atom on the surface of fullerenes is bonded to three carbon neighbors, therefore, is sp2 hybridized. Fullerenes are in the form of a hollow sphere, ellipsoid, tube, and many other shapes. Spherical fullerenes C60, the first fullerene molecule manufactured in 1985, is named as buckminsterfullerene, resembles the balls used in football. Cylindrical fullerenes are known as carbon nanotubes (CNTs). As the discovery of fullerenes came after buckminsterfullerene, the shortened name “fullerene” is used to refer to the family of fullerenes, of which each carbon atom is covalently bonded to three others. The C60 polyhedra are informally called bucky balls. The original method of making the allotrope was to fire a high-power laser at a graphite target. This also produces less stable carbon clusters, such as C70. It can be produced more conveniently using an electric arc between graphite electrodes in an inert gas. The allotrope is soluble in benzene, from which it can be crystallized to give yellow crystals. This form of carbon is also known as fullerite.
Solid C6o exhibits a large number of interesting physical and chemical properties. One of the theoretical predictions was that the C60 molecule itself would have a bulk modulus larger than that of diamond and the soft material under high pressure would become harder than diamond.Fullerene-C60 (C60) is approximately 0.7 nm in diameter. It is a hollow, icosahedrally-shaped, closed-cage structure consisting of 60 sp2 hybridized carbon atoms which can be utilized for the preparation of novel carbon nanomaterials.
The discovery of buckminsterfullerene led to a considerable amount of research into its properties and compounds. Particular interest has been shown in trapping metal ions inside the carbon cage to form enclosure compounds. Buckminsterfullerene itself is often simply called fullerene. The term also applies to derivatives of buckminsterfullerene and to similar cluster (e.g. C70). Carbon structures similar to that in C60 can also form small tubes, known as bucky tubes.
Properties
Buckminsterfullerene is the largest object observed to exhibit wave–particle duality; theoretically every object exhibits this behavior.The compound is stable, withstanding high temperatures and high pressures.C60 undergoes six reversible, one-electron reductions to C6−60, but oxidation is irreversible. The first reduction needs ≈1.0 V (Fc/Fc+), showing that C60 is a moderately effective electron acceptor. C60 tends to avoid having double bonds in the pentagonal rings, which makes electron delocalization poor, and results in C60 not being "superaromatic". C60 behaves very much like an electron deficient alkene and readily reacts with electron rich species.A carbon atom in the C60 molecule can be substituted by a nitrogen or boron atom yielding a C59N or C59B respectively.
History
Fullerenes were first observed in 1985 in the sooty residue left after vaporizing carbon in a helium atmosphere. The discoverers thought the icosahedral structures with exactly 60 unsaturated carbon atoms resembled geodesic domes popularized by famous architect Buckminster Fuller and named them "buckminsterfullerenes" in his honor. The name has since been shortened to "fullerene," but they are sometimes also called "buckyballs."
Cosmetics Uses
Buckyballs, the soccer-shaped molecules discoveredover a decade ago, are made entirelyof carbon atoms linked with unusual chemicalbonds. Fullerenes are soluble carbon molecules that are studied and incorporated into cosmetics for their anti-oxidant and free-radical scavenging properties. Some manufacturers cite significantly greater anti-oxidant potential than vitamins C and e and an ability to retain their antifree radical activity under a variety of external conditions such as heat, strong ultraviolet radiation. They are most commonly used to minimize potential reactions through an interaction with the immune system. Fullerenes are generally incorporated into antiaging and skin rejuvenation formations. The most common form of fullerenes is C60; other forms include C70, C76, and C84. They are the result of nanotechnology and its application in skin care.
Medical Uses
In the medical field, elements such as helium (that can be detected in minute quantities) can be used as chemical tracers in impregnated buckyballs. Water-soluble derivatives of C60 were discovered to exert an inhibition on the three isoforms of nitric oxide synthase, with slightly different potencies.The optical absorption properties of C60 match solar spectrum in a way that suggests that C60-based films could be useful for photovoltaic applications. Because of its high electronic affinity it is one of the most common electron acceptors used in donor/acceptor based solar cells. Conversion efficiencies up to 5.7% have been reported in C60–polymer cells.
Industrial Uses
Fullerenes are a family of molecules that containan even number of carbon atoms in a closedcage.The molecule is a hollow, pure carbonmolecule in which the atoms lie at the verticesof a polyhedron with 12 pentagonal faces andany number (other than one) of hexagonalfaces.The fullerenes were discovered as a consequenceof astrophysically motivated chemicalphysics experiments that were interpreted byusing geodesic architectural concepts.Fullerene chemistry,a field that appears to holdmuch promise for materials development andother applied areas,was born from pure fundamentalscience.
Buckminster fullerene (C60 or fullerene-60),is the archetypal member of the fullerenes.Otherstable members of the fullerene family have similarstructures.The fullerenes can beconsidered,after graphite and diamond,to be thethird well-defined allotrope of carbon.
The fullerenes promise to have synthetic,pharmaceutical, and industrial applications.Derivatives have been found to exhibit fascinatingelectrical and magnetic behavior, in particularsuperconductivity and ferromagnetism.
The properties of fullerene materials that have been determined suggest that there is likely to be a wide range of areas in which the fullerenes or their derivatives will have uses. The facility for acceptance and release of electrons suggests a possible role as a charge carrier in batteries.
Fullerene nanotubes, tiny, tubular carbon fibers, were recently cut into open-ended pipes for the first time. This allows them to be chemically manipulated for use in nanotechnologies and materials. The attachment of molecules to the ends of the pipes lets them serve as means of binding to other chemical groups or surfaces.
The properties of graphite suggest that lubricative as well as tensile and other mechanical properties of the fullerenes are worthy of investigation. Liquid solutions exhibit excellent properties of optical harmonic generation. The high temperature at which superconducting behavior is observed suggests possible applications in microelectronics devices, as does the detection of ferromagnetism in other fullerene derivatives.
The most specific feature of fullerenes is that they are excellent electron acceptors (n-type semiconductors), which are suitable for organic electronic materials with electron carriers. Fullerenes can be used to fabricate polymer/fullerene blend for organic photovoltaics, in which fullerene acts as the n-type semiconductor. Fullerenes are powerful antioxidants, which react readily and at a high rate with free radicals. These free radicals usually lead to cell damage or death. Therefore, fullerenes show great promise in health and personal care applications, in which the prevention of oxidative cell damage or death is desirable. Fullerenes are also used in non-physiological applications, where oxidation and radical processes are destructive (food spoilage, plastics deterioration, metal corrosion). Research interests on fullerenes include also the use of fullerenes to control the neurological damage of diseases such as Alzheimer’s disease and Lou Gehrig’s disease. Fullerenes are used as additives for polymers to create copolymers or composites with specific physical and mechanical properties. Fullerenes can be doped with rubidium and cesium to fabricate superconductors with electron carriers, of which the superconducting transitions occur at more than 30 K.