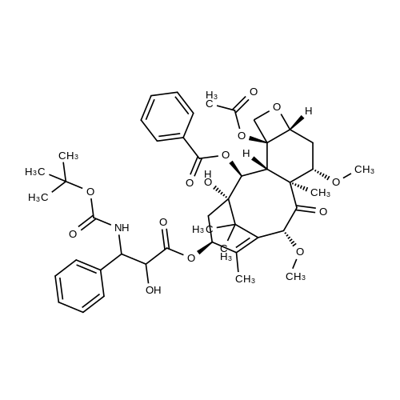
What is Cabazitaxel?
Feb 11,2020
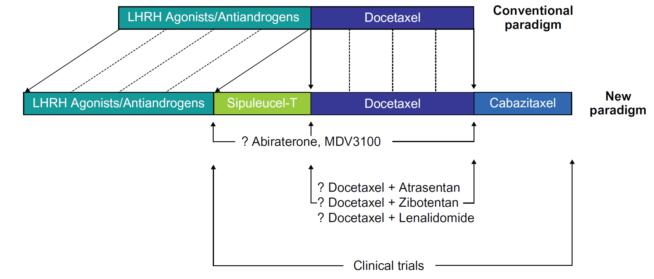
The taxane derivative cabazitaxel (JevtanaR) is approved in the USA and the EU for use in combination with prednisone for the treatment of patients with hormone-refractory metastatic prostate cancer previously treated with a docetaxel-containing regimen.[1]

Cabazitaxel is a semisynthetic taxane derivative that acts as a microtubule inhibitor. It binds to tubulin, promoting the assembly of tubulin into microtubules and inhibiting their disassembly, which results in microtubule stabilization, the inhibition of cell division, cell cycle arrest and the arrest of tumour proliferation. Cabazitaxel demonstrated antitumour activity against advanced human tumours xenografted in mice. As well as being active in docetaxel-sensitive tumours, cabazitaxel showed activity in tumour models insensitive to chemotherapy, including docetaxel. Cabazitaxel also penetrates the blood-brain barrier to a greater extent than docetaxel. [2]
The pharmacokinetic data for cabazitaxel demonstrated dose proportionality, with a high plasma clearance and a long terminal half-life. The very large volume of distribution at steady state suggests extensive penetration into tissues. Of interest, cabazitaxel is able to cross the blood-brain barrier in preclinical models. Cabazitaxel is mainly metabolized by the cytochrome P450 (CYP) enzyme 3A4/5 and to a lesser extent by CYP2C8, suggesting that it has the potential to inhibit CYP3A enzymes. [3]
Cabazitaxel is associated with a manageable and acceptable safety profile. Although, in the TROPIC trial, cabazitaxel treatment was associated with a higher number of deaths during the treatment period as a result of neutropenia and diarrhoea, mandatory provision of well structured contingency plans, including patient education, access to acute specialized care, haematopoietic growth factor support, antimicrobials and supportive care will help to mitigate against these adverse events. [4]
In all, cabazitaxel is a very promising novel antineoplastic agent that has been approved in the US and Europe for the treatment of men with mCRPC whose disease has progressed following treatment with docetaxel-based chemotherapy. The emergence of this new chemotherapeutic agent is very encouraging in an era otherwise dominated by new, biological anticancer agents.
References
1.Oudard S. TROPIC: phase III trial of cabazitaxel for the treatment of metastatic castration-resistant prostate cancer[J]. Future Oncol. 2011, 7(4):497–506.
2.Sartor AO. Progression of metastatic castrate-resistant prostate cancer: impact of therapeutic intervention in the post-docetaxel space[J]. J Hematol Oncol. 2011, 4:18.
3.Tsao CK, Seng S, Oh WK, et al. Clinical development of cabazitaxel for the treatment of castration-resistant prostate cancer[J]. Clin Med Insights Oncol. 2011, 5:163–9.
Dieras V, Lortholary A, Laurence V, et al. Cabazitaxel in patients with advanced solid tumours: results of a phase I and pharmacokinetic study[J]. Eur J Cancer. 2013, 49(1):25–34.
- Related articles
- Related Qustion
See also
Suvorexant (BelsomraR), a first-in-class, orally active dual orexin-1 receptor and orexin-2 receptor antagonist, has been developed by Merck for the treatment of insomnia.....
Feb 10,2020DrugsDiclofenac is a phenylacetic acid derivative belonging to the class of the non-selective non-steroidal anti-inflammatory drugs (NSAIDs). It exhibits analgesic, antipyretic and anti-inflammatory activity.....
Feb 11,2020Antipyretic analgesicsCabazitaxel
183133-96-2You may like
- Cabazitaxel
-
- $1.10 / 1g
- 2024-04-17
- CAS:183133-96-2
- Min. Order: 1g
- Purity: 99.0% min
- Supply Ability: 100 tons min
- Cabazitaxel
-

- $0.00 / 25kg
- 2024-04-12
- CAS:183133-96-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000ton
- Cabazitaxel
-

- $0.00 / 1KG
- 2024-03-16
- CAS:183133-96-2
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg