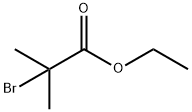
What is Ethyl 2-bromoisobutyrate?
Nov 22,2021
General description
Ethyl 2-bromoisobutyrate, with the CAS No: 600-00-0. This chemical’s molecular formula is C6H11BrO2 and molecular Weight is 195.06g/mol. The boiling point is 161℃ at 760 mmHg. The flash point is greater than 60℃. The density is 1.311g/cm3. The appearance is colorless liquid. It is Easily soluble in ethanol and ether, insoluble in water.


Figure 1 The structure of Ethyl 2-bromoisobutyrate
The synthesis of Ethyl 2-bromoisobutyrate
Method 1: Ethyl isobutyrate (116.1 g, 1 mol) was irradiated and heated to reflux by using a 450-W photolamp, and then 159.8 g (1 mol) of bromine was added under stirring at a rate so that discoloration took place. Stirring was continued for 2 h followed by an immediate distillation of the reaction mixture to give 5


Figure 2 The synthesis of Ethyl 2-bromoisobutyrate.
Method 2:Isobutyric acid was sequentially added to a 500 ml four-necked flask quipped with a stirring slurry and a thermometer: 35.5 g (content: 99.0%, 0.3994 mol), cyclohexane: 300 ml (about 230 g), dibenzoyl peroxide 0.25 g, N-bromosuccinimide: 75.6 g (content: 98.8%, 0.4196 mol). The water separator is fixed on the four-necked flask, and the condenser is fixed on the upper part of the water separator. The stirring was started, and the stirring speed was set to 500 r/min or more. The outer wall of the four-necked bottle is heated in a water bath, and the hot water temperature of the water bath is set to 90 ° C. When the internal temperature of the four-necked bottle reaches about 82 ° C, the reaction bottle begins to boil and vaporize, and the vaporized cyclohexane will react the trace water in the system. Bring the reaction flask out and deposit it on the bottom of the trap.As the reaction progresses, the reaction liquid gradually changes from colorless to pale yellow, and the N-bromosuccinimide originally formed in the bottom of the bottle forms a succinimide suspended in the upper portion of the reaction flask. After refluxing for 3 to 8 hours, the content of the reaction solution was sampled and detected. When the residual isobutyric acid in the reaction solution was less than 1.0%, the reaction was stopped.After the reaction is completed, the outer wall of the reaction flask is cooled with 20 to 30 ° C of cooling water. When the internal temperature reaches below 35 ° C, filter. The filter cake was succinimide and the filtrate was a cyclohexane solution of 2-bromoisobutyric acid.The filtrate was transferred to a 500 ml three-necked flask equipped with a thermometer and a stirring paddle, and the three-necked flask was fixed to the bottom of the distillation column. Add zinc powder to the three-necked flask: 0.8g, stir for 30 minutes, the color of the material in the three-necked bottle will gradually fade. Further, 46% hydrobromic acid: 1.0 g was placed in a three-necked flask, and after stirring for 30 minutes, 33.0 g of ethanol (content: 99.0%, 0.7102 mol) was added.The outer wall of the three-necked flask was heated in a water bath, and the water temperature of the water bath was set to 95 °C. The mixture is stirred, and the whole reflux reaction is heated up. During the reaction, the turbid light component having a higher water content is continuously produced. After 5 to 10 hours of reflux dehydration reaction, the content of the material in the reaction flask was sampled and detected, and when the content of 2-brominated isobutyric acid was less than 3.5%, the reaction was stopped.The outer wall of the reaction flask was cooled with cooling water of 20 to 30 ° C. When the internal temperature was less than 35 ° C, stirring was stopped, the contents of the reaction flask were transferred to a separatory funnel, and the reaction liquid was washed three times with 200 ml of deionized water. After washing, the contents of the separatory funnel were transferred to a 500 ml three-necked flask equipped with a thermometer and a stirring paddle. The three-necked flask was fixed to a recovery device, and the outer wall of the three-necked flask was heated in a water bath, the water temperature of the water bath was set to 80 ° C, the internal temperature of the reaction flask was controlled to be ≤ 60 ° C, and the vacuum was ≥ 0.06 MPa to recover n-hexane under reduced pressure. After completion of the recovery, 69.4 g of a colorless transparent ethyl 2-bromoisobutyrate (content: 96.6%, yield: 86.1%) was obtained.
The effect of the organic solvents for ethyl-2-bromoisobutyrate
The effect of the organic solvents on the conversion of ethyl-2-bromoisobutyrate is given in Figure 8. The order of the reactivity of reactants (ethyl-2-bromoisobutyrate) in these organic solvents is: di-n-butyl ether > n-hexane > chlorobenzene > benzene > dichloromethane. The order of the polarity (or dielectric constant) of the organic solvents is: dichloromethane > chlorobenzene > di-n-butyl ether > benzene >n-hexane. It is clear that the conversion of ethyl-2-bromoisobutyrate does not correspond to the order of dielectric constants of the organic solvents. The values of k1 and k4 estimated from these organic solvents are given in Table III. The yield of ethyl-2-hydroxyisobutyrate and 2-hydroxyisobutyric acid are both obtained using dichloromethane, benzene, and chlorobenzene as the organic solvents. However, the product of 2-bromoisobutyric acid is not obtained by using n-hexane and di-n-butyl ether as the organic solvents (k4 ¼ 0).


Figure 4 Effect of organic solvents on the conversion of hydrolyzing ethyl-2-bromoisobutyrate; 0.026 mole of ethyl-2-bromoisobutyrate, 0.078 mole of NaOH, 50 mL of water, 50 mL of chlorobenzene, 800 rpm, 30C
Reference
[1] Schank K , Frisch A , Zwanenburg B . .alpha.-Oxo sulfones. 4. Correction of a pretended. alpha.-oxo sulfone by an unambiguous synthesis of the revised structure[J]. The Journal of Organic Chemistry, 1983.
[2] üNSAL, A?IKEL, Elif K , Sevilay T , et al. INDIVIDUAL AND SIMULTANEOUS BIOSORPTION OF CHROMIUM (VI) AND NICKEL (II) ONTO DRIED ACTIVATED SLUDGE[J]. Chemical Engineering Communications, 2004, 191(12):1589-1605.
- Related articles
- Related Qustion
- Ethyl 2-bromoisobutyrate: Overview, Other Properties and Synthesis Feb 26, 2024
Ethyl 2-bromoisobutyrate is a crucial ester compound, synthesized by ethyl alcohol and 2-bromoisobutyric acid, valued for its hydrolysis reactivity and role in polymer synthesis.
- Ethyl 2-bromoisobutyrate: properties, applications and safety Oct 25, 2023
Ethyl 2-bromoisobutyrate is a versatile organic compound used in synthesis and antimicrobial polymer development, but requires careful handling due to its hazardous nature.
Methyl 2-bromo-2-methylpropionate can be used as a free radical initiator to participate in polymerization reactions. With current economic capabilities, it can be mass-produced at a lower cost.....
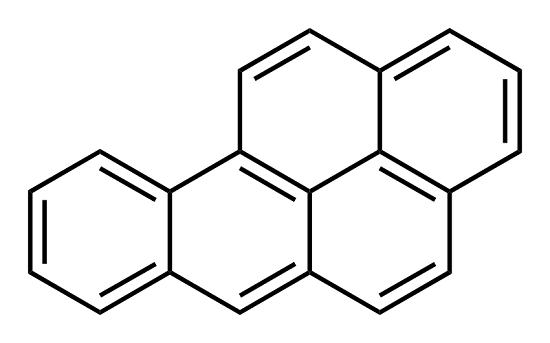
Nov 22,2021Chemical ReagentsBenzo(a)pyrene (BaP) is bioactivated to its carcinogenic form by phase 1 and phase 2 metabolism. As with other polycyclic aromatic hydrocarbons (PAHs), the presence of the ‘bay region’ contributes greatly to the carcinogenicity of BaP. This....
Nov 22,2021Chemical ReagentsEthyl 2-bromoisobutyrate
600-00-0You may like
Ethyl 2-bromoisobutyrate manufacturers
- Ethyl 2-bromoisobutyrate
-

- $30.00 / 1kg
- 2025-06-20
- CAS:600-00-0
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons
- Ethyl 2-bromoisobutyrate
-

- $1.00 / 1KG
- 2025-05-26
- CAS:600-00-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100000kg
- Ethyl 2-bromo-2-methylpropionate
-

- $48.00 / 1KG
- 2023-05-04
- CAS:600-00-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 200tons