What is Methyl 2-bromo-2-methylpropionate?
Nov 22,2021
General description
Methyl 2-bromo-2-methylpropionate, with the CAS No: 23426-63-3. This chemical’s molecular formula is C5H9BrO2 and molecular Weight is 181.03 g/mol. The boiling point is 145.83℃ at 760 mmHg. The flash point is 46.89℃. The density is 1.44 g/cm3.
The refractive index of Methyl 2-bromo-2-methylpropionate is 1.455, the article is flammable and irritating Methyl 2-bromo-2-methylpropionate is a commonly used organic compound. Methyl 2-bromo-2-methylpropionate can be used as a free radical initiator to participate in polymerization reactions. With current economic capabilities, it can be mass-produced at a lower cost. It is used in the chemical industry, especially in the pharmaceutical industry, and can be used as an excellent intermediate. The process is converted into various useful ingredients. Methyl 2-bromo-2-methylpropionate is in liquid form at room temperature, so it usually needs to be packed in plastic drums and must be sealed. The main reason is that it is easy to volatilize flammable gas in an open environment, and when the gas concentration reaches a certain level, it may be ignited, thereby posing risks to storage and transportation. Warehouses storing this substance must be marked with dangerous goods, do not stack with other flammable items, and open flames must be strictly prohibited in the storage environment.
If it is completely pure methyl 2-bromoisobutyrate, the color is transparent, but in the case of industrial production, slight impurities are allowed. At this time, the color will appear slightly yellow. As long as the relevant standards are combined, it will not The use especially in the production environment, is allowed to contain magazines in the raw materials, but if it is a magazine that depends on the manufacturing process or the nature of the finished product, strict testing is required, and raw materials that do not meet the standards can not be used.
In general, methyl 2-bromoisobutyrate is a chemical with relatively stable properties, and the related synthesis process and use process are mature. At the same time, under normal temperature conditions, its toxicity is relatively small, and it will not cause too much danger to the users. However, when high temperature or other special properties of the material are connected, it may decompose and release toxic gases, so proper protection is still needed.


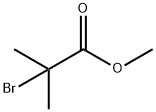
 Figure 1 The structure of Methyl 2-bromo-2-methylpropionate
Figure 1 The structure of Methyl 2-bromo-2-methylpropionate
The synthesis of Methyl 2-bromo-2-methylpropionate
Bromoisobutyric acid (4.17 g, 25 mmol) was dissolved in a solution of concentrated sulfuric acid (0.75 mL) and methanol (50 mL) and stirred under reflux for 2 h. The solution was then cooled to room temperature and concentrated under vacuum. The residue was dissolved in diethyl ether and washed with 10% aqueous sodium bicarbonate, washed with brine, dried under magnesium sulfate, and concentrated again under vacuum. A colorless oil was obtained (4.2 g, 23 mmol, 93% yield) 1H NMR (300 MHz, CDCl3) δ(ppm): 3.78 (s, 3H), 1.93 (s, 6H)


Figure 2 The synthesis of Methyl 2-bromo-2-methylpropionate.


Figure 3 1H-NMR spectrum (CDCl3, 300 MHz) of Methyl 2-bromo-2-methylpropionate
The participating reaction of Methyl 2-bromo-2-methylpropionate
Methyl 2-bromo-2-methylpropionate reacts with zinc and arylglyoxals under conditions of Reformatsky reaction in ether-HMPA mixture to afford 1-aryl-4,4,8,8-tetramethyl-2,6-dioxabicyclo[3.3.0]- octane-3,7-diones in 68-85% yield. Methyl 2-bromopropionate reacts with zinc and 4-bromophenylglyoxal in the same way giving the bicyclic product in 28% yield. The bromination of 1-(4-tolyl)-4,4,8,8-tetramethyl-2,6-dioxabicyclo[3.3.0]octane-3,7-dione with bromosuccinimide results in successive replacement of hydrogens in the tolyl moiety by bromine. The calculation of formation enthalpy for stereoisomers of compounds obtained in approximation predicts the highest stability for chair-type conformation with eclipsed position of substituents at C1 and C5 atoms.
Reference
[1] Hakobyan K , Mcerlean C , M Müllner. Activating ATRP Initiators to Incorporate End-Group Modularity into Photo-RAFT Polymerization[J]. Macromolecules, 2020, 53(23):10357-10365.
[2] üNSAL, A?IKEL, Elif K , Sevilay T , et al. INDIVIDUAL AND SIMULTANEOUS BIOSORPTION OF CHROMIUM (VI) AND NICKEL (II) ONTO DRIED ACTIVATED SLUDGE[J]. Chemical Engineering Communications, 2004, 191(12):1589-1605.
- Related articles
- Related Qustion
Bifenthrin is an off-white to pale tan waxy solid with a very faint slightly sweet odor. Used as a broad spectrum insecticide.....
Nov 22,2021DrugsEthyl 2-bromoisobutyrate, with the CAS No: 600-00-0. This chemical’s molecular formula is C6H11BrO2 and molecular Weight is 195.06g/mol. The boiling point is 161℃ at 760 mmHg.....
Nov 22,2021Chemical ReagentsMethyl 2-bromo-2-methylpropionate
23426-63-3You may like
Methyl 2-bromo-2-methylpropionate manufacturers
- Methyl 2-bromo-2-methylpropionate
-

- $0.00 / 200KG
- 2025-12-05
- CAS:23426-63-3
- Min. Order: 1KG
- Purity: ≥99%
- Supply Ability: 200mt/year
- Methyl 2-bromo-2-methylpropionate
-
- $1.00 / 1kg
- 2025-12-03
- CAS:23426-63-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 mt
- Methyl 2-bromo-2-methylpropionate
-

- $0.00 / 1KG
- 2025-06-27
- CAS:23426-63-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg