Formamide appears as a colorless liquid with a faint odor of ammonia. Denser than water. Freezing point 36°F.
Formamide, a physiological product of N,N-dimethylformamide, was detected in the urine of synthetic leather factory workers. Urinary concns ranged from 7-69 mg/l for workers exposed to N,N-dimethylformamide in the workplace[1].
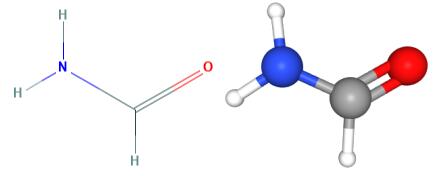
Formamide is the simplest monocarboxylic acid amide, obtained by formal condensation of formic acid with ammonia. The parent of the class of formaldehydes. Formamide has a role as a solvent. Formamide is a monocarboxylic acid amide, a one-carbon compound and a member of formamides. Formamide derives from a formic acid. Formamide is a tautomer of a formimidic acid.Formamide is a metabolite used for biological monitoring of workers exposed to N-N-dimethylformamide (DMF). There is a case of significant association between ever having been exposed to DMF and subsequent development of prostate cancer[2].
Fig 1. Chemical structure formula and three-dimensional structure of Formamide
Formamide is incompatible with strong oxidizers, acids and bases. Sensitive to light. Reacts with water very slowly at room temperature, but rate is accelerated by acids and bases at elevated temperatures. Incompatible with iodine, pyridine and sulfur trioxide. Reacts explosively with furfuryl alcohol, H2O2, Tl(NO3)3·H2O, nitromethane and P2O5. An effective solvent: dissolves casein, glucose, tannins, starch, lignin, polyvinyl alcohol, cellulose acetate, nylon, the chlorides of copper, lead, zinc, tin, cobalt, iron, aluminum and nickel, the acetates of the alkali metals, some inorganic sulfates and nitrates. Attacks copper and brass[3].Formamide is hydrolyzed very slowly at room temperature. Acids, bases and elevated temperatures accelerate the hydrolysis[4].
Formamide's production and use as an intermediate in the chemical industry; to produce heterocyclic compounds, pharmaceuticals, crop protection agents, fungicides, and pesticides; as a solvent in the manufacture and processing of plastics; to produce formic acid; to remove coating from copper conductors; in the spinning of acrylonitrile copolymers; in the antistatic finishing of plastics or formation of conductive coatings on plastic particles; may result in its release to the environment through various waste streams. If released to air, a vapor pressure of 6.1X10-2 mm Hg at 25 °C indicates formamide will exist solely as a vapor in the ambient atmosphere. Vapor-phase formamide will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 8.0 days. If released to soil, formamide is expected to have very high mobility based upon a Koc of 3.6. Volatilization from moist soil surfaces is not expected to be an important fate process based upon an estimated Henry's Law constant of 1.4×10-9 atm-cu m/mole. If released into water, formamide is not expected to adsorb to suspended solids and sediment based upon the Koc. Several biodegradation screening studies have observed significant biodegradation of formamide which suggests that biodegradation may be important. Volatilization from water surfaces is not expected to be an important fate process based upon this compound's estimated Henry's Law constant. An estimated BCF of 3 suggests the potential for bioconcentration in aquatic organisms is low. Hydrolysis is expected to be slow. Occupational exposure to formamide may occur through inhalation and dermal contact with this compound at workplaces where formamide is produced or used.
References
[1]A. Casal Lareo, A. Perico, P. Bavazzano. Biological monitoring of workers exposed to N, N-dimethylformamide[J]. Int Arch Occup Environ Health, 1995, 67(1):47-52.
[2]DcC G W. CHRIS: Hazardous Chemical Data[J]. 1978.
[3]Library W E. National Institute of Environmental Health Sciences[M]. 2010.
[4]Review by: A. E. M. McLean. Toxicity and Metabolism of Industrial Solventsby Ethel Browning[J]. British Journal of Industrial Medicine, 23(3):246.