ホルムアミド 化学特性,用途語,生産方法
外観
無色澄明の液体
性質
ホルムアミドは無色の液体で、常温常圧でアンモニア臭があります。融点は2〜3°C、沸点は210°Cです。アルコールやエーテルと混ざりますが、ベンゼンやクロロホルムには溶けません。
水と任意の割合で混ざり合い、水に溶けないイオン性化合物を溶解できるため、よく溶媒に利用されます。具体的には、グルコース、カゼイン、デンプン、タンニンなどの高分子化合物だけでなく、鉛、銅、鉄などの塩化物のほか、酢酸のアルカリ金属塩を溶かします。
溶解性
水及びエタノールに溶けやすく、ジエチルエーテルに極めて溶けにくい。
解説
[同義異語]ホルムアミド
森北出版「化学辞典(第2版)
用途
研究用の溶剤、試薬
用途
医薬品原料、香料
用途
医薬品、染料、顔料、その他有機合成用中間原料として使用される溶剤、たん白、合成繊維糊料、紙などの処理剤、電気化学工業の電解液
用途
1. DNA塩基配列決定法(マキサム・ギルバート法でのDNA変性液, Dideoxy法での停止液)2. サザンブロット法でのハイブリダイゼーション緩衝液3. ノーザンブロット法でのRNA変性液, ハイブリダイゼーション緩衝液4. S1マッピングでのハイブリダイゼーション緩衝液
構造
ホルムアミドはギ酸由来のアミドです。示性式はHCONH2と表されます。同じくRR'NCHOと表される化合物の1つに、N,N-ジメチルホルムアミド (英: N,N-dimethylformamide) もあり、示性式は(CH3)2NCHOです。なお、分子量は45.04g/molで、密度は1.133g/cm3です。
生化学でホルムアミドは、現在の地球上の生命を維持する能力があります。そのため、水の代替溶媒として提案されています。ホルムアミドはシアン化水素の加水分解によって生じ、双極子モーメントが大きく、溶媒和特性も水に近いです。
合成法
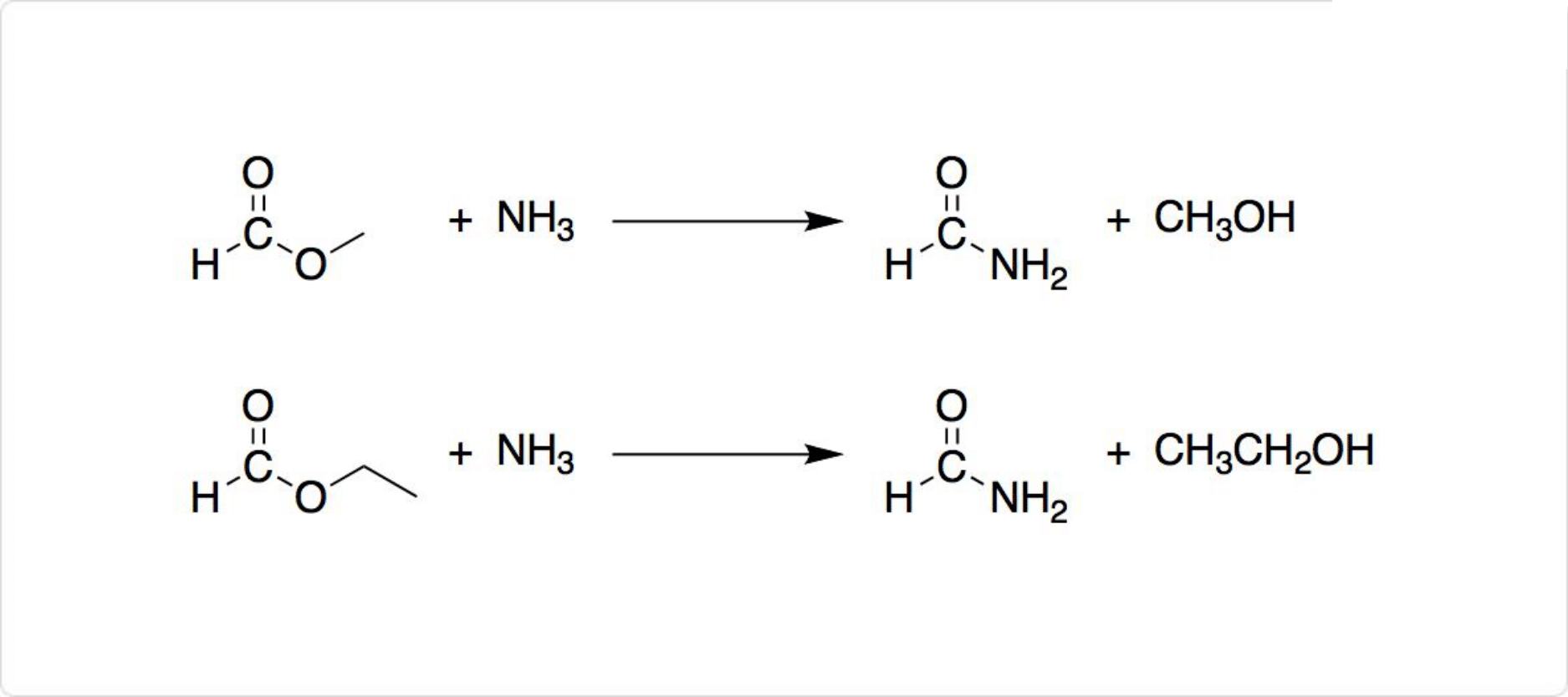
図2. ホルムアミドの合成
ギ酸とを反応させるとギ酸アンモニウム (英: ammonium formate) が生じ、脱水によってホルムアミドを生成可能です。工業的には、 (英: methyl formate) またはギ酸エチル (英: ethyl formate) の、アンモニアによるアミノ分解 (英: aminolysis) が用いられています。
アンモニアのカルボニル化によっても、ホルムアミドを製造可能です。一酸化炭素とメタノールから生成されるギ酸メチルのアンモノリシス (英: ammonolysis) でも得られます。
使用上の注意
吸湿性があり、湿気によって徐々に分解する。
化学的特性
Formamide is a colorless, viscous liquid.
Faint ammonia-like odor.
使用
Formamide destabilizes nucleic acid duplexes and may be used, typically, at a concentration of 50%, in hybridization protocols requiring lower hybridization temperatures.
定義
ChEBI: The simplest monocarboxylic acid amide, obtained by formal condensation of formic acid with ammonia. The parent of the class of formaldehydes.
調製方法
Formamide is produced commercially by two processes (Eberling 1980). In a direct synthesis, ammonia and carbon monoxide react at 100-300 atm and 80-100°C in methanolic sodium methoxide. In the second, a two-stage synthesis, carbon monoxide and methanol form methylformate in the presence of sodium methoxide. The methylformate is treated with liquid or gaseous ammonia at 2-6 atm and 80-100°C.
一般的な説明
A colorless liquid with a faint odor of ammonia. Denser than water. Freezing point 36°F.
空気と水の反応
Hygroscopic. Water soluble.
反応プロフィール
Formamide is incompatible with strong oxidizers, acids and bases. Sensitive to light. Reacts with water very slowly at room temperature, but rate is accelerated by acids and bases at elevated temperatures. Incompatible with iodine, pyridine and sulfur trioxide. Reacts explosively with furfuryl alcohol, H2O2, Tl(NO3)3.H2O, nitromethane and P2O5. An effective solvent: dissolves casein, glucose, tannins, starch, lignin, polyvinyl alcohol, cellulose acetate, nylon, the chlorides of copper, lead, zinc, tin, cobalt, iron, aluminum and nickel, the acetates of the alkali metals, some inorganic sulfates and nitrates. Attacks copper and brass .
危険性
Toxic material. Toxic by skin absorption.
健康ハザード
Formamide is moderately irritating to the skin and mucous membranes (Windholz 1983).
使用用途
ホルムアミドは、凍結防止剤として利用されています。実験に使われる臓器や生体組織を凍結する際には、ホルムアミドを含んだ凍結防止剤を使用可能です。例えば、マウスの精子を凍結保存する場合などが挙げられます。
ホルムアミドは、対象物を固化させて過冷却状態にする「ガラス化化合物」として働きます。ホルムアミドはシアン化水素 (HCN) の工業的製法にも利用可能です。シアン化水素は極めて毒性の高い物質で、殺虫剤や殺鼠剤として利用されています。
また、RNAを脱イオン化するため、ゲル電気泳動 (英: gel electrophoresis) のRNA安定剤としても使用されます。キャピラリー電気泳動では、変性したDNAの一本鎖の安定化に利用可能です。さらに、ビタミンの合成やサルファ薬の製造に利用される以外にも、紙や繊維の柔軟剤にも使われます。
化学反応
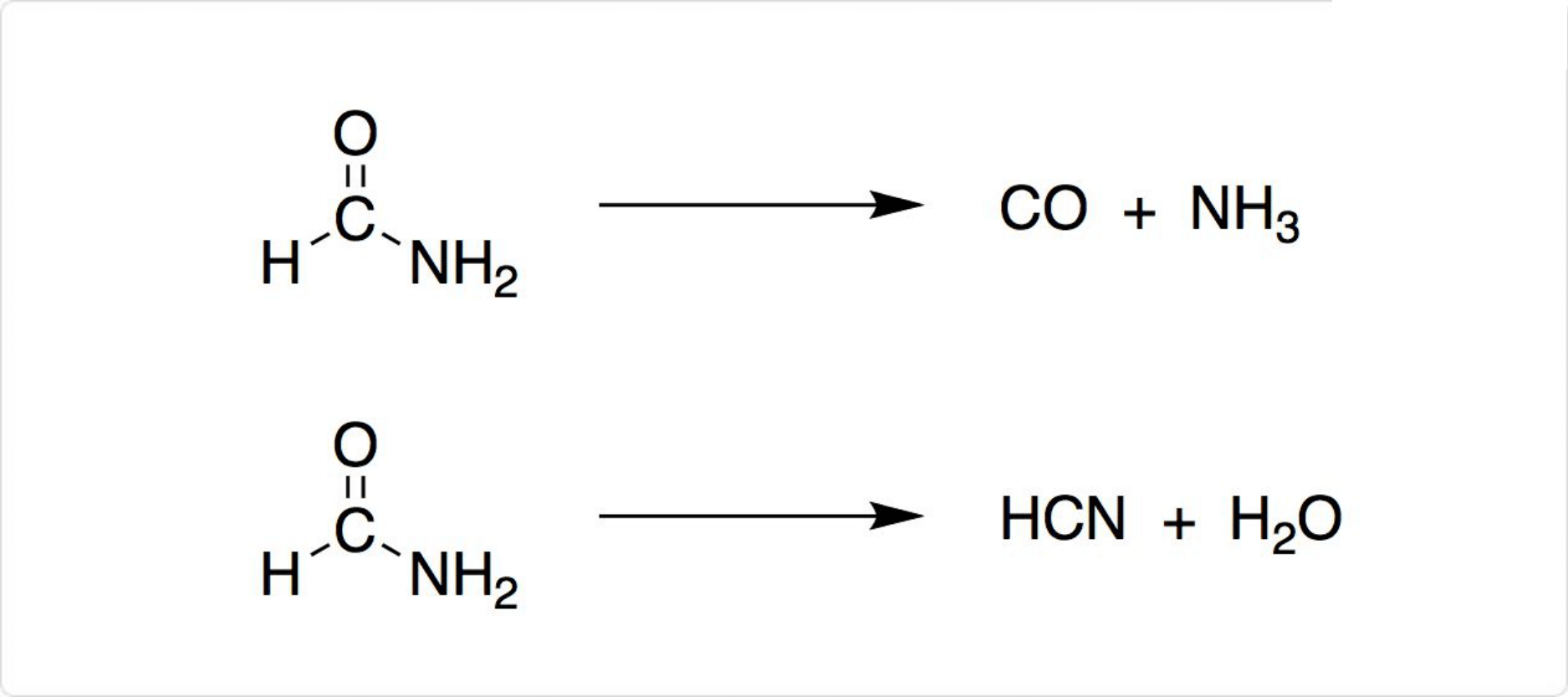
図3. ホルムアミドの反応
180°Cでホルムアミドは、一酸化炭素とアンモニアに分解します。固体酸触媒の存在下では、シアン化水素と水に分解されます。
紫外線の存在下でホルムアミドを加熱すると、微量のグアニン (英: guanine) に変換可能です。
农业用途
Fonnamide is an organic compound containing the amide group -CONH
2. It is made from formic acid or its ester with ammonia. It is also made from ammonia and carbon monoxide.
Formamide is used in making liquid fertilizers for foliar application of nitrogen. For example, a mixture of formamide, urea and ammonium nitrate is used as a solution fertilizer and has a salt-out temperature of 0°C. It contains more than 35% nitrogen, unlike the aqueous formulations of urea, and ammonium nitrate, which have 32 % nitrogen.Formamide is a good solvent for many organic compounds.
工業用途
Formamide is used in the large scale production of formic acid by reaction with inorganic acids, as an intermediate in the chemical industry, as a solvent in the processing of plastics, and as a solvent in felt-tip pens (Eberling 1980).
安全性プロファイル
Poison by skin contact
and subcutaneous routes. Moderately toxic
by ingestion, intraperitoneal, and
intramuscular routes. An irritant to skin,
eyes, and mucous membranes. Experimental
teratogenic and reproductive effects. An eye
irritant. Mutation data reported.
Combustible when exposed to heat or
flame; can react vigorously with oxidizing
materials. Incompatible with 12, pyridine,SO3. When heated to decomposition it emits
toxic fumes of NOx. Has exploded while in
storage.
職業ばく露
Formamide is a powerful solvent. It is
also used as an intermediate in pharmaceutical
manufacture.
環境運命予測
If released to air, formamide will exist solely as a vapor in the
ambient atmosphere. Vapor-phase formamide will be degraded
in the atmosphere by reaction with photochemically produced
hydroxyl radicals. The half-life for this reaction in air is estimated
to be 8.0 days. If released to soil, formamide is expected to have
very high mobility. Volatilization from moist soil surfaces is not
expected to be an important fate process. If released into water,
formamide is not expected to adsorb to suspended solids and
sediment. Several biodegradation screening studies have
observed significant biodegradation of formamide, which
suggests that biodegradation may be important. Volatilization
from water surfaces is not expected to be an important fate
process based upon this compound’s estimated Henry’s law
constant.
代謝
There are only very few reports on the metabolic fate of formamide in the literature. Halsey (1898) found that formamide gave rise to as much urinary formate in the dog as did formic acid, and assumed complete hydrolysis of the amide in vivo. In a study by Bray et al (1949) the hypothesis was tested that formamide undergoes metabolic hydrolysis in rabbits. Acidic substances were titrated after extraction by ether, before and after hydrolysis of urine samples. The ether-soluble acid determined in hydrolyzed urine was assumed to reflect the amount of formamide excreted unchanged. The difference between the amount of amide administered and the total amount excreted unchanged was considered to represent amide which was metabolically hydrolyzed. After administration of 2-4 g per rabbit orally, 39% of the dose was recovered unchanged using this method. When formamide was incubated with rabbit liver extracts or liver slices, only very little hydrolysis was detected by this method.
輸送方法
UN2810 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required.
純化方法
Formamide is easily hydrolysed by acids and bases. It also reacts with peroxides, acid halides, acid anhydrides, esters and (on heating) alcohols, while strong dehydrating agents convert it to a nitrile. It is very hygroscopic. Commercial material often contains acids and ammonium formate. Vorhoek [J Am Chem Soc 58 2577 1956] added some bromothymol blue to formamide and then neutralised it with NaOH before heating to 80-90o under reduced pressure to distil off ammonia and water. The amide is again neutralised and the process is repeated until the liquid remained neutral on heating. Sodium formate is added, and the formamide is concentrated under reduced pressure at 80-90o. The distillate is again neutralised and redistilled. It is then fractionally crystallised in the absence of CO2 and water by partial freezing. Formamide (specific conductance 2 x 10-7 ohm-1 cm-1) of low water content is dried by passage through a column of 3A molecular sieves, then deionized by treatment with a mixed-bed ion-exchange resin loaded with H+ and HCONH-ions (using sodium formamide in formamide)[Notley & Spiro J Chem Soc (B) 362 1966]. [Beilstein 2 IV 45.]
不和合性
Forms hydrocyanic acid with water
solutions. Hygroscopic (absorbs moisture from air).
Incompatible with nonoxidizing mineral acids; strong acids;
ammonia, cresols, iodine, isocyanates, oleum, phenols, pyr idine, sulfur trioxide; oxidizers, iodine, pyridine.
Formamide decomposes on heating @ 180℃ forming
ammonia, water, carbon monoxide and hydrogen cyanide.
Attacks metals, such as aluminum, iron, copper, brass, lead,
and natural rubber. Thermal decomposition may release
deadly hydrogen cyanide. Compounds of the carboxyl
group react with all bases, both inorganic and organic (i.e.,
amines) releasing substantial heat, water and a salt that
may be harmful. Incompatible with arsenic compounds
(releases hydrogen cyanide gas), diazo compounds, dithio carbamates, isocyanates, mercaptans, nitrides, and sulfides
(releasing heat, toxic and possibly flammable gases),
thiosulfates and dithionites (releasing hydrogen sulfate and
oxides of sulfur).
廃棄物の処理
Dissolve in a combustible
solvent and dispose by burning in a furnace equipped with
an alkali scrubber for the exit gases.
ホルムアミド 上流と下流の製品情報
原材料
準備製品
5-(4-Phenyl-phenyl)thieno[2,3-d]pyrimidin-4(3H)-one ,97%
5-メチル-2-ピロール-1-イル-チオフェン-3-カルボン酸
イベルメクチン
6-アミノ-7-メチル-7H-プリン
6-ISO-プロピル-3H-チエノ[2,3-D]ピリミジン-4-オン
(4-OXO-6,7-DIHYDRO-4H,5H-CYCLOPENTA[4,5]THIENO-[2,3-D]PYRIMIDIN-3-YL)-ACETIC ACID
3'-アジド-3'-デオキシチミジン
4-メチルチアゾール
ペプロマイシン
5-Ethyl-6-methylthieno[2,3-d]pyrimidin-4(3H)-one ,97%
1,2,3,5-テトラヒドロ-8-チア-5,7-ジアザ-シクロペンタ[A]インデン-4-オン
N,N-ジエチル-3-(2,4,6-トリメチルフェニルスルホニル)-1H-1,2,4-トリアゾール-1-カルボキサミド
5,6-ジメチルチエノ[2,3-D]ピリミジン-4(3H)-オン
4,5-ジメチルチアゾール
4-クロロ-5,6-ジメチルチエノ[2,3-D]ピリミジン
キサノメリン L-酒石酸 水和物
2-チオフェンカルボキサミド
5-(4-METHOXY-PHENYL)-3H-THIENO[2,3-D]PYRIMIDIN-4-ONE
6-フェニル-3H-チエノ[2,3-D]ピリミジン-4-オン
5-メチル-4-オキソ-3,4-ジヒドロチエノ[2,3-D]-ピリミジン-6-カルボン酸エチル
4-メチル-5-エトキシオキサゾール
6-エチルチエノ[2,3-D]ピリミジン-4(3H)-オン
6-メトキシピリミジン-4-アミン
5,6,7,8-TETRAHYDRO-3H-BENZO[4,5]THIENO[2,3-D]-PYRIMIDIN-4-ONE
2,6-ジメルカプトプリン
酢酸2-(4-メチル-5-チアゾリル)エチル
ラゾキサン
セフォジジム
2,4-ジメトキシベンゼンメタンアミン
ベスナリノン
6-METHYL-3H-THIENO[2,3-D]PYRIMIDIN-4-ONE
4-クロロ-8-フルオロ-5H-ピリミド[5,4-B]インドール
6-TERT-BUTYL-3H-THIENO[2,3-D]PYRIMIDIN-4-ONE
3,5-DIMETHYL-3H-IMIDAZOLE-4-CARBOXYLIC ACID ETHYL ESTER
Β-D-フルクトフラノシル, Α-D-グルコピラノシド, オクタデカノアート
6-ヨード-4-キナゾロン ヨウ化物
3,4-デヒドロ-4-オキソ-7-キンゾリンカルボン酸
methyl 3,4-dihydro-4-oxoquinazoline-7-carboxylate
ジエチルスチルベストロール
5-METHYL-4-OXO-3,4-DIHYDRO-THIENO[2,3-D]PYRIMIDINE-6-CARBOXYLIC ACID