What is Ruxolitinib phosphate?
Feb 10,2020
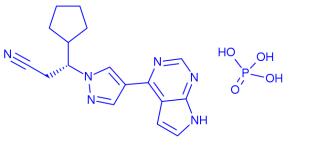
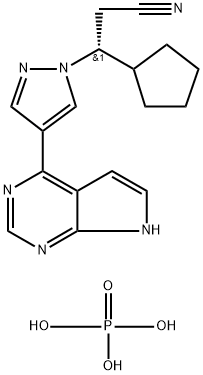
Ruxolitinib, (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3- cyclopentylpropanenitrile phosphate, has a molecular weight of 404.36 kDa. Ruxolitinib is soluble in aqueous solutions at pH 1-8. Ruxolitinib tablets are stable at 20-25°C and tolerate brief exposures to temperatures outside this range, if they stay within 15-30°C. [1]
Ruxolitinib is an oral inhibitor of JAK1 and JAK2, which is approved for the treatment of myeloproliferative neoplasm-associated myelofibrosis, further myeloproliferative neoplasms, polycythemia vera and refractory cancer. In the USA, Canada and EU, ruxolitinib is approved for the treatment of patients with intermediate or high-risk PMF, post-PV MF or post-ET MF. In addition, ruxolitinib recently has been approved for the treatment of patients with PV who have had an inadequate response to or are intolerant of hydroxyurea, based on the results of Phase II and III clinical studies. To date, ruxolitinib has been approved for indications in MF in 83 countries. [2]
Ruxolitinib selectively inhibits the proliferation of JAK2-driven Ba/F3 cells and these effects are correlated with decreased levels of phosphorylated JAK2 and of signal transducer and activator of transcription 5 (STAT5). Treatment with ruxolitinib decreases constitutional symptoms and spleen size in Philadelphia-negative myeloproliferative neoplasms (MPN).
Common side effects of ruxolitinib treatment include anaemia and thrombocytopenia. The decline of circulating erythrocytes following ruxolitinib treatment could result in part from stimulation of eryptosis, the suicidal erythrocyte death characterized by cell shrinkage and cell membrane scrambling with phosphatidylserine translocation to the cell surface. Cellular mechanisms involved in the execution of eryptosis include oxidative stress, Ca2+ entry with increase of cytosolic Ca2+ activity ([Ca2+]i), ceramide, decline of cytosolic ATP, caspases, stimulated activity of casein kinase 1α, Janus-activated kinase JAK3, protein kinase C, and p38 kinase, as well as impaired activity of AMP activated kinase AMPK, cGMP-dependent protein kinase, PAK2 kinase and sorafenib/sunitinib sensitive kinases. [3]
Single doses of ruxolitinib administered orally to healthy volunteers were absorbed rapidly, with times to maximum serum concentration of [less than or equal to]2 h. Ruxolitinib has a short terminal half-life of approximately 3 h for doses up to 100 mg, suggesting that twice-daily (b.i.d.) administration of ruxolitinib is preferable to once-daily dosing. Ruxolitinib is metabolized by CYP3A4 and to a lesser extent by CYP2C9. [4]
In all, Ruxolitinib, a JAK1 and JAK2 inhibitor, is currently the only approved pharmacotherapy that has been shown in randomized controlled trials to be efficacious and safe in the treatment of patients with MF.
References
1.Mesa RA , Green A , Barosi G , Verstovsek S , Vardiman J , Gale RP . MPN-associated myelofibrosis (MPN-MF)[J]. Leuk. Res. 2011, 35 (1):12 - 13
2.Vardiman JW , Thiele J , Arber DA The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes[J] . Blood, 2009, 114 (5):937 - 951
3.Mesa RA , Shields A , Hare T Progressive burden of myelofibrosis in untreated patients: assessment of patient-reported outcomes in patients randomized to placebo in the COMFORT-I study [J]. Leuk. Res. 2013, 37 (8):911 - 916
Lekovic D , Gotic M , Perunicic-Jovanovic M Contribution of comorbidities and grade of bone marrow fibrosis to the prognosis of survival in patients with primary myelofibrosis[J] . Med. Oncol. 2014, 31 (3): 869
- Related articles
- Related Qustion
See also
Carboplatin is a second-generation platinum compound analog with established activity against a broad spectrum of solid tumors including brain tumors, neuroblastoma, rhabdomyosarcoma, and germ cell tumors.....
Feb 10,2020DrugsLetrozole (Femara?) is a nonsteroidal, third-generation aromatase inhibitor administered orally once daily, which has shown efficacy in the treatment of postmenopausal women with early-stage or advanced, hormone-sensitive breast cancer.....
Feb 10,2020DrugsRuxolitinib phosphate
1092939-17-7You may like
Ruxolitinib phosphate manufacturers
- Ruxolitinib phosphate
-

- $44.00 / 5mg
- 2025-04-30
- CAS:1092939-17-7
- Min. Order:
- Purity: 99.91%
- Supply Ability: 10g
- Ruxolitinib Phosphate
-

- $0.00 / 1kg
- 2025-04-02
- CAS:1092939-17-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000kg
- Ruxolitinib Phosphate
-

- $0.00 / 1g
- 2025-01-13
- CAS:1092939-17-7
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month