Active Pharmaceutical Ingredients (API), popularly speaking, are the raw materials of medicines, only pharmaceutical raw materials are processed into pharmaceutical preparations , can they become medicines available for clinical use, so drugs we usually eat are the finished drugs through processing. Active Pharmaceutical Ingredients based on its sources can be divided into two major categories ,including chemical synthetic drugs and natural chemical drugs. Chemical synthetic drugs can be divided into organic synthetic drugs and inorganic synthetic drugs. Inorganic synthetic drugs are inorganic compounds ( very few is element), such as aluminum hydroxide, magnesium trisilicate which are used for the treatment of gastric and duodenal ulcers ; organic synthetic drugs are mainly composed of drugs made by basic organic chemical raw materials, through a series of organic chemical reactions (such as aspirin, chloramphenicol, caffeine, etc.). Natural chemical drugs ,based on its sources,can be divided into two categories including biochemical drugs and plant chemical drugs. Antibiotics are generally made by the microbial fermentation, which belongs to the biochemistry category. A variety of semi-synthetic antibiotics occurs in recent years,which are biosynthesis and chemical synthesis combining products.Among active Pharmaceutical Ingredients, the organic synthetic drugs varieties, yields and values have the largest proportion,which are the main pillars of the chemical and pharmaceutical industries. The quality of active Pharmaceutical Ingredients decides whether the formulation is good or bad , so its quality standards are very strict ,countries in the world have developed national pharmacopoeia standards and strict quality control methods for its widely used active Pharmaceutical ingredients.
What is terbinafine used for?
Oral and topical treatment of dermatophytoses and onychomycoses. Terbinafine also shows activity in the topical treatment of pityriasis versicolor and cutanous candidiasis.
Mar 16,2024 APIWhat is tolnaftate ointment used for?
The indications for tolnaftate are defined by its narrow spectrum and limited to the treatment of dermatophytoses. Tolnaftate is effective when topically administered several times daily over a period
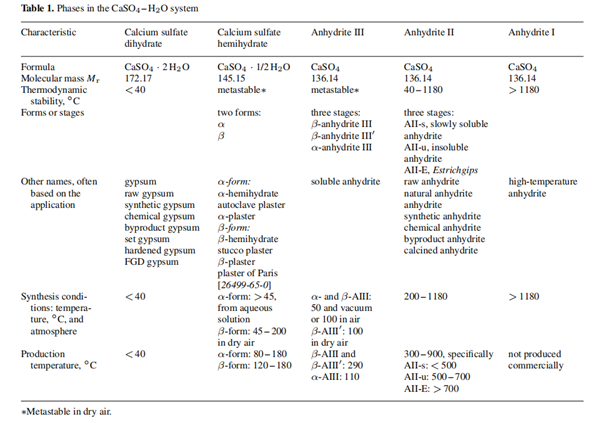
Mar 16,2024 APIThe CaSO4 – H2O System: Physical properties and Laboratory Synthesis
The CaSO4– H2O system is characterized by five solid phases.
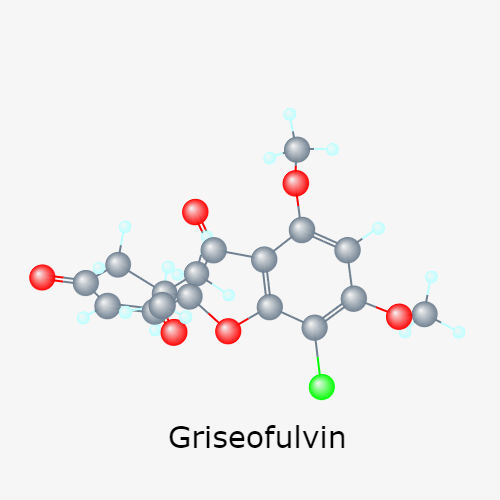
Mar 16,2024 APIWhat is griseofulvin used for?
The indications for griseofulvin are dermatophytoses and onychomycoses which do not respond to topical therapy.
Mar 16,2024 APIWhat is nystatin used to treat?
Nystatin A1 is especially indicated in the therapy of cutaneous and mucocutaneous infections caused by pathogenic yeasts.
Mar 16,2024 APIDoes natamycin have side effects?
When applied topically, natamycin is highly effective and well tolerated in the treatment of cutaneous and mucocutaneous candidoses. Local irritations and allergic reactions may occur in rare cases an
Mar 16,2024 APIWhat is amphotericin B mainly used for?
Amphotericin B is a broad-spectrum polyene antimycotic with activity against pathogenic yeasts, moulds, dimorphic fungi, and in vitro also against dermatophytes.
Mar 16,2024 APIWhat is a terconazole used for?
The triazole antimycotic terconazole was first synthesized at Janssen Pharmaceuticals. Terconazole is a broad-spectrum triazole antimycotic with activity against most species of pathogenic fungi.
Mar 16,2024 APIWhat is clotrimazole used to treat?
The imidazole antimycotic clotrimazole is used to treat mycoses of the skin induced or sustained by fungi such as dermatophytes, yeasts, and chromomycetes.
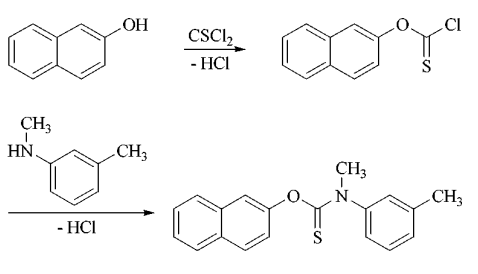
Mar 16,2024 APIWhat is bifonazole used for?
Bifonazoleis a imidazole antimycotic bifonazole used to treat mycoses of the skin induced or sustained by fungi such as dermatophytes, yeasts, and also chromomycetes.
Mar 16,2024 API