Active Pharmaceutical Ingredients (API), popularly speaking, are the raw materials of medicines, only pharmaceutical raw materials are processed into pharmaceutical preparations , can they become medicines available for clinical use, so drugs we usually eat are the finished drugs through processing. Active Pharmaceutical Ingredients based on its sources can be divided into two major categories ,including chemical synthetic drugs and natural chemical drugs. Chemical synthetic drugs can be divided into organic synthetic drugs and inorganic synthetic drugs. Inorganic synthetic drugs are inorganic compounds ( very few is element), such as aluminum hydroxide, magnesium trisilicate which are used for the treatment of gastric and duodenal ulcers ; organic synthetic drugs are mainly composed of drugs made by basic organic chemical raw materials, through a series of organic chemical reactions (such as aspirin, chloramphenicol, caffeine, etc.). Natural chemical drugs ,based on its sources,can be divided into two categories including biochemical drugs and plant chemical drugs. Antibiotics are generally made by the microbial fermentation, which belongs to the biochemistry category. A variety of semi-synthetic antibiotics occurs in recent years,which are biosynthesis and chemical synthesis combining products.Among active Pharmaceutical Ingredients, the organic synthetic drugs varieties, yields and values have the largest proportion,which are the main pillars of the chemical and pharmaceutical industries. The quality of active Pharmaceutical Ingredients decides whether the formulation is good or bad , so its quality standards are very strict ,countries in the world have developed national pharmacopoeia standards and strict quality control methods for its widely used active Pharmaceutical ingredients.
Function of D-serine in kidney
D-serine has therapeutic potential in kidney diseases due to its ability to promote cellular proliferation and address immune-related factors contributing to damage.
Jan 15,2024 APILanolin: A Compound for Alleviating Sore and Cracked Nipples during Lactation
Lanolin effectively reduces nipple pain and improves healing during lactation without negative effects on breastfeeding, mastitis, or exclusivity rate.
Jan 15,2024 APIAzilsartan as a Potent Antihypertensive Drug with Possible Pleiotropic Cardiometabolic Effects
Azilsartan, a prodrug and ARB, effectively treats hypertension by inhibiting AT1 receptors. It may provide metabolic benefits and potential organ protection, but further research is required.
Jan 15,2024 APIMecobalamin: mechanism, pharmacokinetics and metabolism
Mecobalamin treats B12 deficiency-related neuropathy, lowers homocysteine levels to reduce cardiovascular disease risks and shows high efficacy with various administration routes.
Jan 15,2024 APIIndigo: physicochemical properties, production pathways and biological production
Indigo is a dye derived from natural plants and can be produced through plant-based or microbial fermentation pathways, with genetic engineering offering improvements for sustainable production.
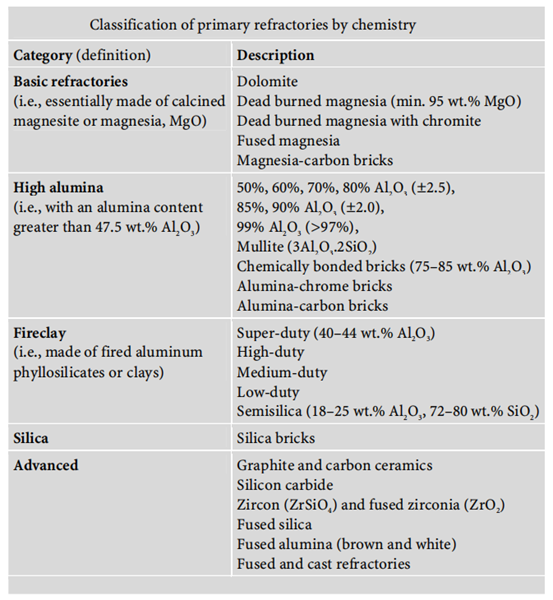
Jan 15,2024 APIRefractories: Classification and Properties
The classification of refractories can be approached in a number of different ways: chemical composition, type of applications, or operating temperature range.
Jan 12,2024 APISilicon Carbide: General Properties; Preparation; Grades
Silicon carbide (SiC), relative molar mass 40.097, is an important advanced ceramic with a high melting point (2830°C), a high thermal conductivity (135 Wm–1K–1), and extremely high Mohs hardness of 9
Jan 12,2024 APIWhat are the effects of Pyridine on human health and the environment?
Pyridine is a flammable, colourless liquid, six-membered heterocyclic compound with an unpleasant odour. Pyridine can cause first degree burns when exposed for short periods of time.
Jan 12,2024 APIBoron nitride-based nanocomposite hydrogels: properties and medical applications
Boron nitride-based nanocomposite hydrogels offer biodegradable, biocompatible properties for wound healing, cancer treatment, and controlled drug delivery in medical applications.
Jan 12,2024 APIFullerene C60: biological features, ROS generation and quenching and ROS-dependent biological effects
Fullerene C60 have antioxidant properties, generating singlet oxygen but limited ROS-quenching capacity, with promise in biomedical applications for their cytotoxic and cytoprotective effects.
Jan 12,2024 API