Active Pharmaceutical Ingredients (API), popularly speaking, are the raw materials of medicines, only pharmaceutical raw materials are processed into pharmaceutical preparations , can they become medicines available for clinical use, so drugs we usually eat are the finished drugs through processing. Active Pharmaceutical Ingredients based on its sources can be divided into two major categories ,including chemical synthetic drugs and natural chemical drugs. Chemical synthetic drugs can be divided into organic synthetic drugs and inorganic synthetic drugs. Inorganic synthetic drugs are inorganic compounds ( very few is element), such as aluminum hydroxide, magnesium trisilicate which are used for the treatment of gastric and duodenal ulcers ; organic synthetic drugs are mainly composed of drugs made by basic organic chemical raw materials, through a series of organic chemical reactions (such as aspirin, chloramphenicol, caffeine, etc.). Natural chemical drugs ,based on its sources,can be divided into two categories including biochemical drugs and plant chemical drugs. Antibiotics are generally made by the microbial fermentation, which belongs to the biochemistry category. A variety of semi-synthetic antibiotics occurs in recent years,which are biosynthesis and chemical synthesis combining products.Among active Pharmaceutical Ingredients, the organic synthetic drugs varieties, yields and values have the largest proportion,which are the main pillars of the chemical and pharmaceutical industries. The quality of active Pharmaceutical Ingredients decides whether the formulation is good or bad , so its quality standards are very strict ,countries in the world have developed national pharmacopoeia standards and strict quality control methods for its widely used active Pharmaceutical ingredients.
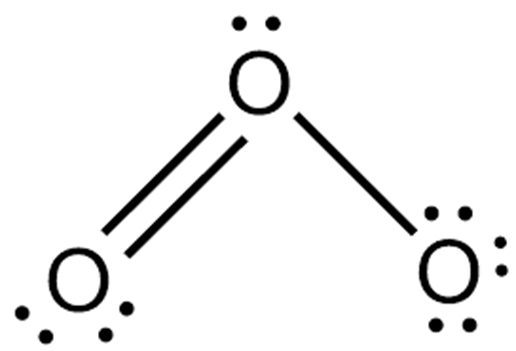
How the Ozone lewis structure is formed
he Lewis structure of O3 also consists of three oxygen atoms bonded at an angle of 116 degrees. There is one double bond and one single bond between the oxygen atoms (O).
Nov 21,2023 APIEthyl levulinate: properties, applications and safety
Ethyl levulinate's properties and safety make it a promising and eco-friendly option for diverse industrial uses.
Nov 21,2023 APILetrozole: pharmacodynamics, pharmacokinetics and mechanism of action
Letrozole is a highly effective aromatase inhibitor used in hormone-responsive breast cancer treatment, exhibiting rapid absorption, effective estrogen reduction, and minimal systemic absorption.
Nov 21,2023 APILysergol: activities, applications and toxicity
Lysergol exhibits diverse activities and potential applications, including antidepressant and antimicrobial effects, antioxidant properties, and potential anticancer effects.
Nov 21,2023 APIBaloxavir: pharmacodynamics, pharmacokinetics and clinical applications
Baloxavir is a potent antiviral targeting influenza, offering rapid relief and potential for future treatments.
Nov 21,2023 APIUV absorber-928: properties, applications and safety
UV absorber-928 offers broad-spectrum UV protection for polymers and coatings, but requires careful handling for environmental safety.
Nov 21,2023 API(S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl: properties, applications and safety
(S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl is a versatile chiral ligand with excellent coordinating ability, toxicity precautions needed, and potential in photocatalysis and cancer research.
Nov 21,2023 API1,2,3-Triacetyl-5-deoxy-D-ribose: properties, applications and safety
1,2,3-Triacetyl-5-deoxy-D-ribose is a white powder used in organic synthesis and pharmaceutical research, with applications in nucleoside synthesis and as a protecting group.
Nov 21,2023 APICan Olivetol treat obesity?
Olivetol was able to inhibit the storage of excess fat in the liver caused by obesity (steatohepatitis) by lowering the levels of liver enzymes.
Nov 20,2023 APIRole of Pyridoxal phosphate in neonatal epileptic encephalopathy
PLP may be effective in treating neonatal epileptic encephalopathy (NEE).
Nov 20,2023 API