Inorganic chemicals is the shortened form of inorganic chemical industry and is an important branch of the chemical industry with natural resources and industrial by-products as raw materials for the production of sulfuric acid, nitric acid, hydrochloric acid, phosphoric acid, soda ash, caustic soda, synthetic ammonia, fertilizer and inorganic salts, etc. This includes sulfuric acid industry, soda industry, the chloro-alkali industry, synthetic ammonia industry, fertilizer industry and mineral industry. Its broad definition also includes the production of inorganic non-metallic materials and fine inorganic product such as ceramics and inorganic pigment. The main raw material of inorganic chemical products are mineral product including sulfur, sodium, phosphorus, potassium and calcium and coal, oil, gas, and air, water and so on. Inorganic chemicals can be traced back to the ancient process of ceramics, alchemy, brewing, dyeing at thousands of years ago. Although with small scale, backward technology and pure manual manipulation, but it is the prototype of inorganic chemicals. For thousands of years, due to the low productivity, it gets slow development. Until the 18th century, it had developed rapidly. In the middle of 18th century, Britain had first applied lead chamber method using saltpeter and sulfur as raw materials to produce sulfuric acid. In 1783, Lu Bulan (France) proposed the soda method using sodium chloride, sulfuric acid, coal as raw materials. In the latter half of the 18th century, the modern chemical industry taking inorganic chemical industry as the main content had began to emerge. In 1841, people began the production of phosphate fertilizer; In 1965 Belgian Solvay realized the industrialization of ammonia soda for production of soda; with the rise of preparing potassium industry in 1870; In 1890, people began to use electrolytic approach for making Cl2 and caustic soda; In 1913, people had achieved the catalytic synthesis
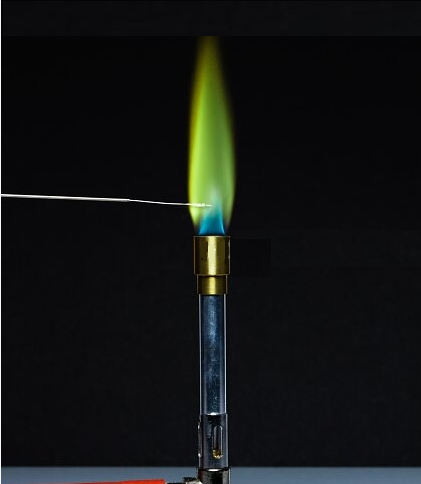
What is Iron flame test
The positive result of a flame test for iron (Fe) produces a golden color. This is due to the excitation of electrons in the sample by the flame's heat. As these electrons lose their energy, they emit
Feb 2,2024 Inorganic chemistryCobalt and Flame Test
Cobalt is a lustrous very hard silvery metal belonging to a group called the "transition metals". It is always used in flame test, to eliminate the effects of sodium.
Jan 26,2024 Inorganic chemistryWhat is the flame colour of rubidium?
Rubidium is a soft, silvery-white metallic element of the alkali metals group (Group 1). It is one of the most electropositive and alkaline elements. Its flame is yellowish-violet.
Jan 26,2024 Inorganic chemistryFlame color of Copper sulfate and Principle of flame tests
Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula CuSO4.
Jan 25,2024 Inorganic chemistryFlame test and chemical principles of silver
Silver do not produce a characteristic flame test color. There are several possible explanations for this.
Jan 25,2024 Inorganic chemistryWhy is Boron green in a flame?
In a flame test, the element Boron emits EM radiation that is predominantly green in color.
Jan 25,2024 Inorganic chemistryWhy does Barium Chloride burn green?
When burned, barium chloride produces a bright green color in flames. It is also known to be hygroscopic, meaning it absorbs moisture from the air.
Jan 25,2024 Inorganic chemistryWhy Is a Propane Gas Flame Blue?
A blue flame means complete combustion of the gas. With complete combustion, LPG (Propane) burns with a blue flame. Pure hydrocarbons like methane, propane, butane and ethane gases also burn with a bl
Jan 25,2024 Inorganic chemistryWhat Color Does Boric Acid Burn?
A very pale green color is imparted to the flame by boron in boric acid. Because, Boric Acid is a boron containing compound Any boron-containing compound will cause flames to emit a green colour.
Jan 25,2024 Inorganic chemistryBarium: Discovery, Sources and the Use in Fireworks
Barium is a soft silvery metal, always found combined with other elements. Barium emits a green flame when it burns, which is used to create the green effect in fireworks.
Jan 24,2024 Inorganic chemistry