Inorganic chemicals is the shortened form of inorganic chemical industry and is an important branch of the chemical industry with natural resources and industrial by-products as raw materials for the production of sulfuric acid, nitric acid, hydrochloric acid, phosphoric acid, soda ash, caustic soda, synthetic ammonia, fertilizer and inorganic salts, etc. This includes sulfuric acid industry, soda industry, the chloro-alkali industry, synthetic ammonia industry, fertilizer industry and mineral industry. Its broad definition also includes the production of inorganic non-metallic materials and fine inorganic product such as ceramics and inorganic pigment. The main raw material of inorganic chemical products are mineral product including sulfur, sodium, phosphorus, potassium and calcium and coal, oil, gas, and air, water and so on. Inorganic chemicals can be traced back to the ancient process of ceramics, alchemy, brewing, dyeing at thousands of years ago. Although with small scale, backward technology and pure manual manipulation, but it is the prototype of inorganic chemicals. For thousands of years, due to the low productivity, it gets slow development. Until the 18th century, it had developed rapidly. In the middle of 18th century, Britain had first applied lead chamber method using saltpeter and sulfur as raw materials to produce sulfuric acid. In 1783, Lu Bulan (France) proposed the soda method using sodium chloride, sulfuric acid, coal as raw materials. In the latter half of the 18th century, the modern chemical industry taking inorganic chemical industry as the main content had began to emerge. In 1841, people began the production of phosphate fertilizer; In 1965 Belgian Solvay realized the industrialization of ammonia soda for production of soda; with the rise of preparing potassium industry in 1870; In 1890, people began to use electrolytic approach for making Cl2 and caustic soda; In 1913, people had achieved the catalytic synthesis
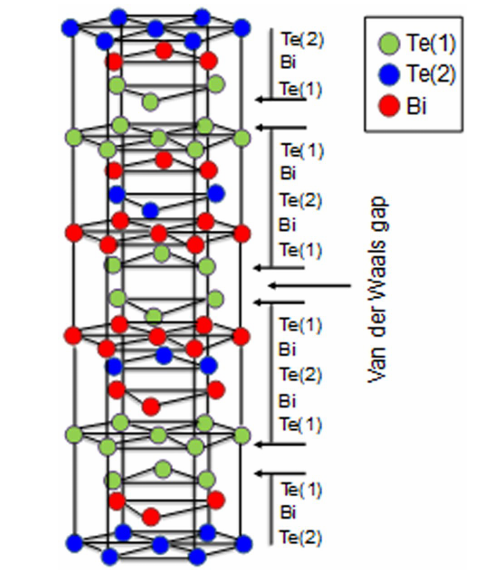
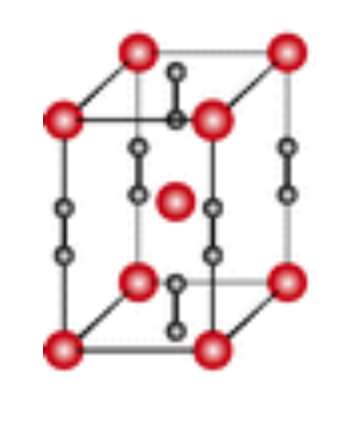
Crystal structure and Uses of Bismuth telluride
Bismuth telluride is a narrow-gap layered semiconductor with a trigonal unit cell.
Mar 26,2024 Inorganic chemistryThe crystal strucure of Aluminum arsenide
Aluminum arsenide (AlAs) is instrumental in various high-tech applications ranging from high-speed electronics, optoelectronics, and integrated circuits to solar energy and quantum technology.
Mar 25,2024 Inorganic chemistryUses and Properties of Chromium Nitride
Chromium Nitride is an extremely hard, inert, thin film coating that is applied primarily to precision metal parts.
Mar 25,2024 Inorganic chemistryGallium telluride:Crystal structure,Synthesis,Toxicity
Gallium telluride is an odorless, black, brittle crystalline solid and is a semiconductor of the III-VI type that crystallizes in a lattice structure.
Mar 25,2024 Inorganic chemistryCrystal structure and Uses of Iron boride
Iron boride has properties of ceramics such as high hardness, and properties of metal properties, such as thermal conductivity and electrical conductivity.
Mar 22,2024 Inorganic chemistryThe crystal structure of uranium dicarbide
Uranium carbide (UC) and uranium dicarbide (UC2) are two carbides of uranium which can be used as nuclear fuels. Uranium sesquicarbide (U2C3) is another carbide of uranium.
Mar 22,2024 Inorganic chemistryIs PbS Soluble in water?
It is a metal sulfide that, like other sulfides in its neighboring spots in the periodic table, is also only sparingly soluble in water.
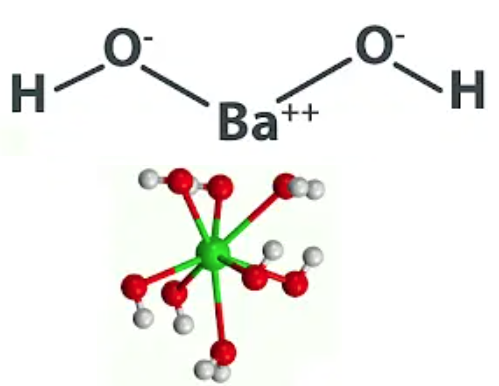
Mar 22,2024 Inorganic chemistryQ:Is Ba(OH)2 a strong base?
A:As the metal it is made of (barium) sits at the bottom of Group II, its hydroxide is considerably strong and comparable to Group I alkalis.
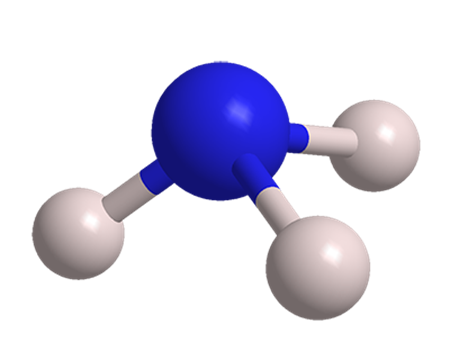
Mar 21,2024 Inorganic chemistryWhat is the conjugate base of NH3?Is it amphotericital?
The conjugate base of NH3 is NH2- which is known as the azide anion.
Mar 21,2024 Inorganic chemistryWhere Can We Find Krypton in Everyday Life?
Krypton was discovered that the gas obtained by evaporating the components of liquid air could also be used to fill incandescent lamps. To this day, it still exists in our daily lives.
Mar 21,2024 Inorganic chemistry