| Identification | Back Directory | [Name]
BAY 80-6946 (Copanlisib) | [CAS]
1032568-63-0 | [Synonyms]
CS-2321
Aliqopa)
BAY80-6946
Copanlisib
Copanlisib base
Copanlisib free base
Copanlisib(BAY80-6946)
BAY 80-6946 (BAY80-6946
BAY 80-6946 (Copanlisib)
BAY 80-6946 (Copanlisib) USP/EP/BP
Copanlisib, 98%, an ATP-competitive selective class-I PI3 kinases inhibitor
7-methoxy-8-(3-morpholinopropoxy)-2,3-dihydroimidazo[1,2-c]quinazolin-5-amine
BAY 80-6946; BAY80-6946; BAY-80-6946; BAY806946; BAY-806946; BAY 806946; COPANLISIB FREE BASE
2-Amino-N-[2,3-dihydro-7-methoxy-8-[3-(4-morpholinyl)propoxy]imidazo[1,2-c]quinazolin-5-yl]-5-pyrimidinecarboxamide
5-Pyrimidinecarboxamide, 2-amino-N-[2,3-dihydro-7-methoxy-8-[3-(4-morpholinyl)propoxy]imidazo[1,2-c]quinazolin-5-yl]- | [Molecular Formula]
C23H28N8O4 | [MDL Number]
MFCD18633201 | [MOL File]
1032568-63-0.mol | [Molecular Weight]
480.52 |
| Chemical Properties | Back Directory | [density ]
1.51 | [storage temp. ]
Store at -20°C | [solubility ]
insoluble in DMSO; insoluble in H2O; insoluble in EtOH | [form ]
solid | [pka]
8.42±0.20(Predicted) | [color ]
Off-white to light brown |
| Hazard Information | Back Directory | [Uses]
BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. | [Definition]
ChEBI: Copanlisib is an imidazoquinazoline that is 2,3-dihydroimidazo[1,2-c]quinazoline substituted by (2-aminopyrimidine-5-carbonyl)amino, methoxy, and 3-(morpholin-4-yl)propoxy groups at positions 5, 7 and 8, respectively. It is a intravenous pan-class I PI3K inhibitor used for the treatment of relapsed follicular lymphoma in patients who have received at least 2 prior systemic therapies. It has a role as an EC 2.7.1.137 (phosphatidylinositol 3-kinase) inhibitor, an antineoplastic agent and an apoptosis inducer. It is a member of morpholines, an aromatic ether, a diether, a tertiary amino compound, a secondary carboxamide, a pyrimidinecarboxamide, an aminopyrimidine and an imidazoquinazoline. | [Brand name]
Aliqopa | [General Description]
Class: lipid kinase;
Treatment: FL (IV infusion);
Other name: BAY 80-6946;
Elimination half-life = 52 h (IV);
Protein binding = 84.2% | [Pharmacokinetics]
Copanlisib has poor oral bioavailability due to
low permeability and poor solubility, and therefore, it
is not suitable for an oral medication. Consequently,
it was developed as an IV drug. IV infusion of
copanlisib resulted in rapid distribution throughout
the body and a prolonged elimination half-life (52 h). | [Synthesis]
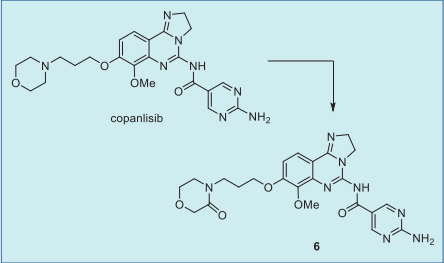
Example 10: Step A9: Preparation of copanlisib (10). 1250 g of 7-methoxy-8-[3-(morpholin-4-yl)propoxy]-2,3-dihydroimidazo[1,2-c]quinazolin-5-amine, 20.3 kg of N,N-dimethylformamide, 531 g of 2-amino-pyrimidine-5-carboxylic acid, 425 g of N,N-dimethylaminopyridine, and 1000 g of N-[3-(dimethylamino)propyl]-N'-ethylcarbodi N'-ethylcarbodiimide hydrochloride were mixed and the reaction was stirred at room temperature for 17 hours. After completion of the reaction, the reaction mixture was filtered and the solid was collected. The solid was washed with N,N-dimethylformamide followed by ethanol and dried at 50 °C to give 1.6 kg of the target product in 96% yield. The resulting product was directly converted to its dihydrochloride form. | [target]
pan-PI3K | [Metabolism]
Copanlisib was the predominant component in
human plasma, accounting for 84% of total
radioactivity AUC, and the morpholinone metabolite
6 was the only circulating metabolite (about 5%).
 | [References]
[1] Patent: WO2016/71435, 2016, A2. Location in patent: Page/Page column 55
[2] Patent: WO2016/71426, 2016, A1. Location in patent: Page/Page column 37
[3] Patent: WO2008/70150, 2008, A1. Location in patent: Page/Page column 85
[4] ChemMedChem, 2016, p. 1517 - 1530 |
| Questions And Answer | Back Directory | [Binding Mode]
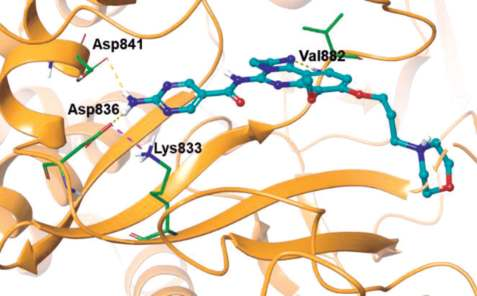
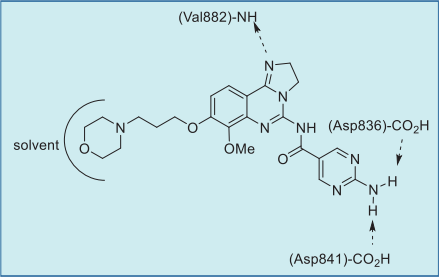
As shown in the co-crystal structure of
copanlisib bound to PI3Kγ, the inhibitor binds with
only one critical hydrogen bond to the amide NH of
Val882 in the adenine pocket, employing the
imidazoline N1 nitrogen (Figs. 1 and 2). In addition,
the C5 aminopyrimidine group fills the affinity pocket,
forming hydrogen bonds with two carboxylic
residues of Asp836 and Asp841 through the amino
group. Finally, the solvent exposed morpholine lies
over Trp812, presumably providing additional
attractive molecular contacts.
  |
|
|