| Chemical Properties | Back Directory | [Boiling point ]
597.7±50.0 °C(Predicted) | [storage temp. ]
4°C, protect from light | [solubility ]
DMSO : 50 mg/mL (186.42 mM; ultrasonic and warming and heat to 60°C) | [form ]
Solid | [color ]
White to off-white | [InChI]
InChI=1S/C9H12F2N2O5/c10-9(11)6(16)4(3-14)18-7(9)13-2-1-5(15)12-8(13)17/h4,6-7,14,16H,1-3H2,(H,12,15,17)/t4-,6-,7-/m1/s1 | [InChIKey]
VQSLORATCUBFCL-QPPQHZFASA-N | [SMILES]
OC[C@H]1O[C@@H](N2CCC(=O)NC2=O)[C@](F)(F)[C@@H]1O |
| Hazard Information | Back Directory | [Uses]
Cedazuridine (E7727) (Compound 7a) is an orally active cytidine deaminase (CDA) inhibitor with an IC50 value of 0.4 μM. Cedazuridine can be used for cancer research[1]. | [Mechanism of action]
Cedazuridine increases the half-life of decitabine by inhibiting cytosine deaminase, thereby improving the efficacy of combination therapy and having a better therapeutic effect than decitabine alone.
| [Synthesis]
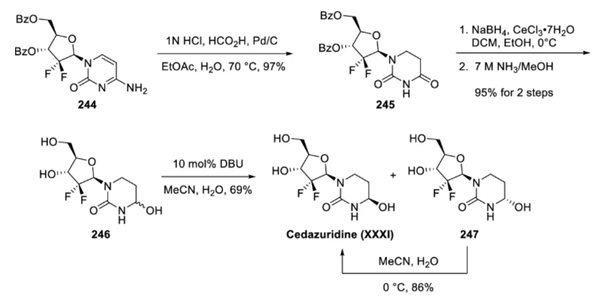
The synthesis began with the readily available protected gemcitabine precursor 244. The 6-aminopyridine was converted to the corresponding dihydrouracil 245 in 97% yield via acid-mediated transfer hydrogenation. Reduction under Luche conditions followed by treatment with methanolic ammonia reduced the amide carbonyl group and removed the two phenoxyester protecting groups to afford dihydrouracil 246 as a mixture of two diastereoisomers. Treatment of 246 with a catalytic amount of DBU in aqueous acetonitrile afforded a diastereomeric mixture in which cedaruridine and its cyclic amino alcohol 247 were present in a 9:1 ratio. Reconstitution of the undesired diastereomer 247 in a cold acetonitrile/water mixture (5:1) afforded cedaruridine in 86% yield.
| [in vivo]
Cedazuridine (3 mg/kg; p.o.; daily for 7 days) in combination with 2.5 mg/kg AZA shows tumor regression in mice MOLM-13 CDX and PDX models[2]. | Animal Model: | Female NSGS mice, 6–8 weeks old, human cell line-derived (CDX) and primary patient-derived xenograft (PDX) models[2] | | Dosage: | 3 mg/kg | | Administration: | Oral administration, in combination with 2.5 mg/kg AZA, daily for 7 days | | Result: | Led to reduction of leukemic expansion in combination with AZA in a cell line-derived xenograft transplantation, and exhibited preliminary safety and efcacy in a primary AML PDX model. |
| Animal Model: | NSGS male mice[2] | | Dosage: | 1, 3, 10 and 30 mg/kg | | Administration: | Oral, in combination with 2.5 mg/kg AZA (Pharmacokinetic Studies) | | Result: | Dose-dependently increased the AUC of oral AZA and in comparison to dosing of standard i.p. AZA. |
| [storage]
4°C, protect from light | [References]
[1] Ferraris D, et al. Design, synthesis, and pharmacological evaluation of fluorinated tetrahydrouridine derivatives as inhibitors of cytidine deaminase. J Med Chem. 2014 Mar 27; 57(6):2582-8. DOI:10.1021/jm401856k
[2] Ramsey H E, et al. Oral azacitidine and cedazuridine approximate parenteral azacitidine efficacy in murine model. Targeted Oncology, 2020, 15(2): 231-240. |
|
|