| Identification | Back Directory | [Name]
2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile | [CAS]
1187595-85-2 | [Synonyms]
Baricitinib-010
[1-(ethylsulfonyl)azetidin-3-ylidene]acetonitrile
2-(1-ETHYLSULFONY)AZETIDIN-3-YLIDENE)ACETONITRILE
(1-Ethanesulfonyl-azetidin-3-ylidene)-acetonitrile
2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile
2-[1-(Ethylsulfonyl)-3-azetidinylidene]acetonitrile
2-[1-(ethanesulfonyl)azetidin-3-ylidene]acetonitrile
Acetonitrile, 2-[1-(ethylsulfonyl)-3-azetidinylidene]-
2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile ISO 9001:2015 REACH
Baricitinib intermediates 2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile | [EINECS(EC#)]
258-714-6 | [Molecular Formula]
C7H10N2O2S | [MDL Number]
MFCD22987586 | [MOL File]
1187595-85-2.mol | [Molecular Weight]
186.232 |
| Chemical Properties | Back Directory | [Melting point ]
67-69°C | [Boiling point ]
360.8±52.0 °C(Predicted) | [density ]
1.33±0.1 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
-8.49±0.20(Predicted) | [color ]
Off-White to Light Yellow | [InChI]
InChI=1S/C7H10N2O2S/c1-2-12(10,11)9-5-7(6-9)3-4-8/h3H,2,5-6H2,1H3 | [InChIKey]
HQUIOHSYUKWGOM-UHFFFAOYSA-N | [SMILES]
C(#N)/C=C1\CN(S(CC)(=O)=O)C\1 |
| Hazard Information | Back Directory | [Uses]
2-(1-(Ethylsulfonyl)azetidin-3-ylidene)acetonitrile is an intermediate in the synthesis of Baricitinib, a JAK 1 and 2 inhibitor used in the treatment of rheumatoid arthritis. | [Synthesis]
2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile is synthesized by
first treating azetidine-3-ol hydrochloride with an equimolar
equivalent of an alkanesulfonyl chloride, preferably ethanesulfonyl
chloride, to give l-ethylsulfonylazetidin-3-ol. Preferably, the
reaction is performed in a biphasic solution comprising a mixture of an
organic phase and a aqueous phase, preferably THF with an aqueous
solution which is basic, while maintaining the solution at room
temperature or a temperature slightly below room temperature, preferably
20 °C. The reaction is followed to completion using standard monitoring
techniques. Typically, the reaction is complete within 1 to 5 hours.
The organic layer is removed, preferably by distillation, and the
aqueous layer is extracted with an appropriate solvent such as toluene,
p-cymene, and CPME. Preferably the extraction solvent is toluene.
Alternatively, the toluene extractions can be excluded if
recrystallization of 2-(1-(ethylsulfonyl)azetidin-3-ylidene)acetonitrile is performed.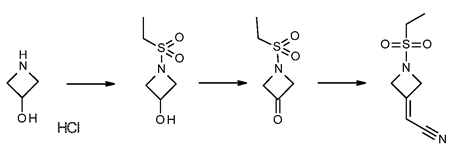
| [References]
[1] Patent: WO2016/125080, 2016, A2. Location in patent: Page/Page column 11
[2] Patent: US2009/233903, 2009, A1. Location in patent: Page/Page column 63-64
[3] Patent: WO2013/40863, 2013, A1. Location in patent: Page/Page column 109-110
[4] Patent: US2016/333015, 2016, A1. Location in patent: Paragraph 0055; 0057; 0134; 0136 |
|
|