| Identification | Back Directory | [Name]
Tazemetostat hydrobromide (JAN/USAN) | [CAS]
1467052-75-0 | [Synonyms]
E-7438 hydrobromide
EPZ-6438 hydrobromide
Tazemetostat hydrobromide
Tazemetostat hydrobromide (JAN/USAN)
Tazemetostat hydrobromide (EPZ6438 or E7438) | [Molecular Formula]
C34H45BrN4O4 | [MDL Number]
MFCD32201156 | [MOL File]
1467052-75-0.mol | [Molecular Weight]
653.66 |
| Chemical Properties | Back Directory | [storage temp. ]
Store at -20°C | [solubility ]
DMSO: 100 mg/mL (152.99 mM) | [InChIKey]
UQRICAQPWZSJNF-UHFFFAOYSA-N | [SMILES]
N(C1CCOCC1)(C1C=C(C2C=CC(CN3CCOCC3)=CC=2)C=C(C(=O)NCC2C(NC(C)=CC=2C)=O)C=1C)CC.Br |
| Hazard Information | Back Directory | [Uses]
Tazemetostat Hydrobromide is developed by Epizyme for the treatment of relapsed or refractory follicular lymphoma.
| [Brand name]
Tazverik
| [Mechanism of action]
Tazemetostat hydrobromide inhibits EZH2, affecting the epigenetic regulation of tumor cells, thereby inhibiting tumor growth.
| [Synthesis]
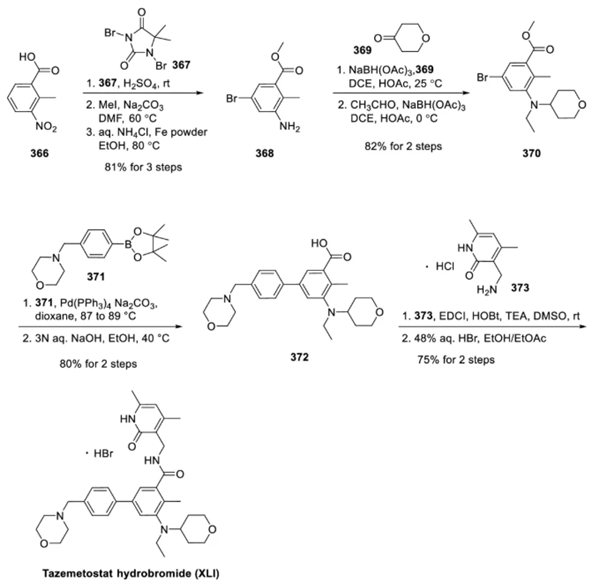
Starting from 2-methyl-3-nitrobenzoic acid (366), dibromohydantoin (367) was used for bromination in sulfuric acid. Aniline 368 was generated through esterification and nitro group reduction in an overall yield of 81%. Aniline 368 was converted to dialkylarylamine 370 by two reductive amination reactions. The first reductive amination was performed with 4-oxoketone (369) and the second reductive amination was performed with acetaldehyde. The yield from 368 to 370 was 82%. Dialkylarylamine 370 was subjected to Suzuki coupling reaction with boric acid ester 371 and then saponification reaction to obtain compound 372 in two steps in an overall yield of 80%. The carboxylic acid of 372 was amide bonded with amine 373 under conventional conditions. Finally, the product was treated with aqueous hydrogen bromide to obtain tazostat hydrobromide in an overall yield of 75%.
| [storage]
Store at -20°C |
|
|