| Identification | More | [Name]
(CYCLOPROPYLMETHYL)TRIPHENYLPHOSPHONIUM BROMIDE | [CAS]
14799-82-7 | [Synonyms]
(CYCLOPROPYLMETHYL)TRIPHENYLPHOSPHONIUM BROMIDE
(CYCLOPROPYLMETHYL)TRIPHENYLPHOSPHONIUM BROMIDE, 98+% | [EINECS(EC#)]
238-862-7 | [Molecular Formula]
C22H22BrP | [MDL Number]
MFCD00051878 | [Molecular Weight]
397.29 | [MOL File]
14799-82-7.mol |
| Chemical Properties | Back Directory | [Melting point ]
182-183°C | [storage temp. ]
Inert atmosphere,Room Temperature | [Appearance]
White to off-white Solid | [Sensitive ]
Hygroscopic | [BRN ]
4068302 | [InChI]
InChI=1S/C22H22P.BrH/c1-4-10-20(11-5-1)23(18-19-16-17-19,21-12-6-2-7-13-21)22-14-8-3-9-15-22;/h1-15,19H,16-18H2;1H/q+1;/p-1 | [InChIKey]
WFQSHRSBITUSIB-UHFFFAOYSA-M | [SMILES]
[P+](CC1CC1)(C1C=CC=CC=1)(C1C=CC=CC=1)C1C=CC=CC=1.[Br-] | [CAS DataBase Reference]
14799-82-7(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Synthesis]
Triphenylphosphine (3.9 g, 14.8 mmoles) was placed into a 300
mL round bottom flask, equipped with a condenser which was cooled
with tap water. Dry benzene (100 mL) (distilled over
sodium/benzophenone) was cannulated into the reaction vessel, and
a positive pressure of argon and magnetic stirring were maintained
throughout the reaction sequence.
Cyclopropylmethyl bromide (2.0 g, 14.8 mmoles) was added to
the solution via syringe. The reaction mixture was heated at reflux
for 10 days producing insoluble cyclopropylmethyltriphenylphosphonium bromide.
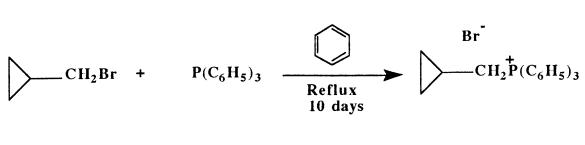 | [References]
[1] Chemical Communications, 2017, vol. 53, # 24, p. 3497 - 3500
[2] Chemical Communications, 2017, vol. 53, # 47, p. 6327 - 6330
[3] Journal of the American Chemical Society, 2009, vol. 131, # 36, p. 12918 - 12920
[4] Liebigs Annalen der Chemie, 1993, # 3, p. 231 - 236
[5] Journal of Organic Chemistry, 1993, vol. 58, # 2, p. 438 - 443 |
|
|