| Identification | Back Directory | [Name]
ADRENOMEDULLIN (RAT) | [CAS]
161383-47-7 | [Synonyms]
ADRENOMEDULLIN
ADRENOMEDULLIN (RAT)
Adrenomedullin (rat) trifluoroacetate salt
Adrenomedullin (rat)/ADM (1-50) (rat), Adrenomedullin (1-50), rat
TYR-ARG-GLN-SER-MET-ASN-GLN-GLY-SER-ARG-SER-THR-GLY-CYS-ARG-PHE-GLY-THR -CYS-THR-MET-GLN-LYS-LEU-ALA-HIS-GLN-ILE-TYR-GLN-PHE-THR-ASP-LYS-ASP-LYS-ASP-GLY-MET-ALA-PRO-ARG-ASN-LYS-ILE-SER-PRO-GLN-GLY-TYR-NH2
H-TYR-ARG-GLN-SER-MET-ASN-GLN-GLY-SER-ARG-SER-THR-GLY-CYS-ARG-PHE-GLY-THR-CYS-THR-MET-GLN-LYS-LEU-ALA-HIS-GLN-ILE-TYR-GLN-PHE-THR-ASP-LYS-ASP-LYS-ASP-GLY-MET-ALA-PRO-ARG-ASN-LYS-ILE-SER-PRO-GLN-GLY-THR-NH2
H-TYR-ARG-GLN-SER-MET-ASN-GLN-GLY-SER-ARG-SER-THR-GLY-CYS-ARG-PHE-GLY-THR-CYS-THR-MET-GLN-LYS-LEU-ALA-HIS-GLN-ILE-TYR-GLN-PHE-THR-ASP-LYS-ASP-LYS-ASP-GLY-MET-ALA-PRO-ARG-ASN-LYS-ILE-SER-PRO-GLN-GLY-TYR-NH2
Adrenomedullin (rat) trifluoroacetate salt H-Tyr-Arg-Gln-Ser-Met-Asn-Gln-Gly-Ser-Arg-Ser-Thr-Gly-Cys-Arg-Phe-Gly-Thr-Cys-Thr-Met-Gln-Lys-Leu-Ala-His-Gln-Ile-Tyr-Gln-Phe-Thr-Asp-Lys-Asp-Lys-Asp-Gly-Met-Ala-Pro-Arg-Asn-Lys-Ile-Ser-Pro-Gln-Gly-Tyr-NH2 trifluoroacetate salt (Disulfide bond) | [Molecular Formula]
C242H381N77O75S5 | [MDL Number]
MFCD00675049 |
| Questions And Answer | Back Directory | [Synthesis and release]
The AM synthesis and release are stimulated by
interleukin-1 (IL-1β), tumor necrosis factor-α (TNF-α),
and lipopolysaccharide (LPS). AM production is
increased by inflammatory cytokines and NO, possibly
mediated by NF-IL6. The expression of ADM is also
induced by hypoxia, ischemia, and oxidative stress. Several conflicting results have been reported regarding the
effect of mechanical stimuli on ADM expression, but the
existence of consensus sequences for the shear stress
responsive element (SSRE) in ADM suggests that AM
synthesis is influenced by these stimuli. AM secretion
from vascular endothelial cells and smooth muscle cells
is stimulated by various substances such as angiotensin
II, thyroid hormone, and steroid hormones, including
aldosterone and glucocorticoids. | [Receptors]
A functional AM receptor is derived from the
calcitonin receptor-like receptor (CLR), whose phenotype
is determined by coexpression with receptor
activity-modifying proteins (RAMPs). The coexpression
of CLR with RAMP2 or RAMP3 produces a receptor
for AM, whereas coexpression with RAMP1 results in
the CGRP receptor. RAMP2 and RAMP3 are indistinguishable in terms of AM binding, and the differential
roles of the CLR/RAMP2 and CLR/RAMP3 receptors
have not been fully clarified. CLR is a seventransmembrane-domain GPCR consisting of 474–548 aa
residues in mammals; it shares 55% sequence identity
with the CT receptor. RAMP is a single-transmembrane
accessory protein that regulates the activities of several
GPCRs. Besides contributing to receptor specificity,
RAMPs are required for the transportation of CLRs from
the endoplasmic reticulum to the plasma membrane.
Three types of RAMPs consisting of 148–175 aa residues
exist in mammals, and five types have been identified in
teleost fish. A functional AM receptor requires another
accessory protein, the receptor component protein (RCP). | [Biological functions]
AM has a wide range of biological functions. Receptor
expression is detected in the vascular smooth muscle cells, vascular endothelial cells, microvessels, heart, lung,
spleen, fat, and kidney. The main functions of AM are
vasodilation, hypotension, angiogenesis, and the regulation of fluid and electrolyte homeostasis. AM is also
synthesized in the hypothalamus and induces oxytocin
release. In nonmammalian species, AM decreases blood
pressure by intravenous injection in eels. | [Clinical implications]
AM is linked to a considerable number of diseases
such as hypertension, congestive heart failure, ischemic
heart injury, pulmonary hypertension, sepsis, cancers,
renal impairment, and diabetes. Elevated plasma levels
of AM are useful in assessing the progression of these
diseases. AM has cardioprotective and vasoprotective
roles in pathophysiological conditions; therefore, the
therapeutic use of AM is considered to be promising in
both acute-phase disorders and chronic diseases. Circulating AM is also increased after tissue transplantation,
suggesting its protective role against oxidative damage.
AM is abundantly expressed in tumor cells and is considered to be involved in carcinogenesis, the promotion of
tumor proliferation, angiogenesis, and the inhibition of
apoptosis. |
| Chemical Properties | Back Directory | [storage temp. ]
−20°C | [form ]
Solid | [color ]
White to off-white | [Sequence]
H-Tyr-Arg-Gln-Ser-Met-Asn-Gln-Gly-Ser-Arg-Ser-Thr-Gly-Cys-Arg-Phe-Gly-Thr-Cys-Thr-Met-Gln-Lys-Leu-Ala-His-Gln-Ile-Tyr-Gln-Phe-Thr-Asp-Lys-Asp-Lys-Asp-Gly-Met-Ala-Pro-Arg-Asn-Lys-Ile-Ser-Pro-Gln-Gly-Tyr-NH2(Disulfide bond) |
| Hazard Information | Back Directory | [Description]
AM is a potent hypotensive peptide synthesized in the
vasculature and various tissues. It is a promising drug target
for hypertension and cardiac diseases. AM was isolated and identified from human pheochromocytoma tissue in 1993 by monitoring the activity
the elevating intracellular cAMP in rat platelets. | [Uses]
Adrenomedullin (rat) is an effective vasodilator peptide. Adrenomedullin is actively secreted by endothelial cells (EC) and vascular smooth muscle cells (VSMC)[1]. | [Structure and conformation]
Human preproAM consists of 185 aa residues and contains a 21-aa residue signal peptide. AM shares the structural homology of an
intramolecular ring structure and has C-terminal amidation with the calcitonin gene-related peptide (CGRP) and
amylin. Therefore, AM is considered to be a member of
the CGRP family. The sequence identities between AM
and other members are not high, including 20% in
humans. ProAM includes a unique sequence of 20 aa residues followed by a typical amidation signal, Gly-Lys-Arg. This peptide with an amidated C-terminus is termed
proadrenomedullin N-terminal 20 peptide (PAMP). AM
is conserved among various vertebrate species, including
teleost fish, though the sequence identities are low among
species. The sequence of the disulfide ring region and the
following C-terminal region are highly conserved in tetrapods, but they vary in teleost fish.
Teleost fish possess two types of paralogous am genes:
am1 and am4. A mature human AM consists of 52 aa residues and
contains a ring structure formed by the intramolecular
disulfide bond between cysteine-16 and cysteine-21.
The tyrosine residue of the carboxyl end is amidated
according to the subsequent amidation signal.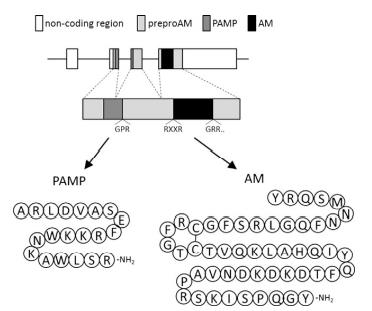 |
|
| Company Name: |
BOC Sciences
|
| Tel: |
1-631-485-4226; 16314854226 |
| Website: |
https://www.bocsci.com |
|