| Identification | More | [Name]
PIMOZIDE | [CAS]
2062-78-4 | [Synonyms]
1-[1-(4,4-BIS[4-FLUOROPHENYL]BUTYL)-4-PIPERIDINYL]-1,3-DIHYDRO-2H-BENZ-IMIDAZOL-2-ONE
PIMOZIDE
1-(1-(4,4-bis(p-fluorophenyl)butyl)-4-piperidyl)-2-benzimidazolinon
1-(1-(4,4-bis(p-fluorophenyl)butyl)-4-piperidyl)-2-benzimidazolinone
1-(4,4-bis(p-fluorophenyl)butyl)-4-(2-oxo-1-benzimidazolinyl)piperidine
mcn-jr-6238
orap
3-(1-(4,4-bis(4-fluorophenyl)butyl)-4-piperidyl)-1H-benzimidazol-2-one
2H-Benzimidazol-2-one, 1-1-4,4-bis(4-fluorophenyl)butyl-4-piperidinyl-1,3-dihydro-
OPIRAN
1-[1-[4,4-Bis(4-fluorophenyl)butyl]-4-piperidinyl]-1,3-dihydro-2H-benzimidazole-2-one
1-[1-[4,4-Bis(4-fluorophenyl)butyl]-4-piperidyl]-1,3-dihydro-2H-benzimidazol-2-one
R 6238 | [EINECS(EC#)]
218-171-7 | [Molecular Formula]
C28H29F2N3O | [MDL Number]
MFCD00055081 | [Molecular Weight]
461.55 | [MOL File]
2062-78-4.mol |
| Chemical Properties | Back Directory | [Appearance]
White or almost white powder. | [Melting point ]
214-218° | [density ]
1.1763 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
DMSO: 18 mg/mL
| [form ]
White solid | [pka]
7.32(at 25℃) | [color ]
Off-White to Light Orange | [Water Solubility ]
2.9mg/L(30 ºC) | [InChIKey]
YVUQSNJEYSNKRX-UHFFFAOYSA-N | [SMILES]
O=C1NC2=CC=CC=C2N1C3CCN(CCCC(C4=CC=C(F)C=C4)C5=CC=C(F)C=C5)CC3 | [CAS DataBase Reference]
2062-78-4(CAS DataBase Reference) |
| Questions And Answer | Back Directory | [Pharmacological action]
In terms of pharmacological action, pimozide is similar to haloperidol. It is used in hospitals as well as in outpatient settings for supportive therapy of patients suffering from schizophrenia, paranoid conditions, and mental and neurotic disorders with paranoid characteristics. It is unfit for use in severe psychoses because it does not possess psychomotor-sedative action. It is used for treating patients who suffer from Turretts’s syndrome. |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed. | [Safety Statements ]
S36:Wear suitable protective clothing . | [RIDADR ]
3249 | [WGK Germany ]
3
| [RTECS ]
DE1750000
| [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
2933399090 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Acute Tox. 4 Oral |
| Hazard Information | Back Directory | [Description]
Pimozide is a dopamine receptor antagonist (Kis = 2.4, 0.3, and 1.8 nM for D2, D3, and D4 receptors, respectively).1 It also binds to eight additional receptors (Kds = 25-3,100 nM for the human receptors) and inhibits the voltage-gated sodium channel Nav1.2 and the voltage-gated potassium channel Kv11.1 (IC50s = 42 and 340 nM, respectively).2,3,4 Pimozide (0.5, 1, and 2 mg/kg) decreases the number of licks and reduces fluid intake of a sweetened solution in rats.5 It decreases the number of threats and attacks and increases immobility time in the neutral arena aggression test, indicating increased passiveness, in male mice when administered at a dose of 0.75 mg/kg for 10 days.6 Formulations containing pimozide have been used in the treatment of Tourette syndrome. | [Chemical Properties]
White or almost white powder. | [Originator]
Orap ,Janssen, W. Germany ,1971 | [Uses]
antipsychotic | [Uses]
Pimozide is a D2 dopamine receptor antagonist that binds to the cloned 5-HT7 receptor with high affinity (1,2,3,4). Pimozide is also a Ca2+ channel antagonist. Pimozide is used as an antipsychotic. | [Uses]
Used for the suppression of motor and phonic tics in patients with Tourette's Disorder who have failed to respond satisfactorily to standard treatment. | [Definition]
ChEBI: A member of the class of benzimidazoles that is 1,3-dihydro-2H-benzimidazol-2-one in which one of the nitrogens is substituted by a piperidin-4-yl group, which in turn is substituted on the nitrogen by a 4,4-bis(p-fluorophen
l)butyl group. | [Manufacturing Process]
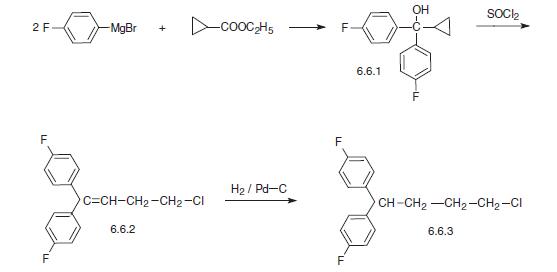
To a solution of 130 parts cyclopropyl-di-(4-fluorophenyl)-carbinol in 240 parts benzene are added dropwise 43 parts thionyl chloride. The whole is refluxed until no more gas is evolved. The reaction mixture is then evaporated. The residue is distilled in vacuo, yielding 4-chloro1,1-di-(4-fluorophenyl)-1-butene, boiling point 165°C to 167°C at 6 mm pressure; nD20: 1.5698; d2020:1.2151.
A solution of 61 parts 4-chloro-1,1-di-(4-fluorophenyl)-1-butene in 400 parts 2-propanol is hydrogenated at normal pressure and at room temperature in the presence of 5.5 parts palladium-on-charcoal catalyst 10% (exothermic reaction: temperature rises to about 30°C). After the calculated amount of hydrogen is taken up, hydrogenation is stopped. The catalyst is filtered off and the filtrate is evaporated. The oily residue is distilled in vacuo, yielding 1chloro-4,4-di-(4-fluorophenyl)-butane, boiling point 166°C to 168°C at 6 mm pressure; nD20: 1.5425; d2020:1.2039.
To a mixture of 4.4 parts of 4-(2-oxo-1-benzimidazolinyl)-piperidine, 3.3 parts sodium carbonate, a few crystals of potassium iodide in 200 parts 4-methyl-2pentanone are added portionwise 6.2 parts 1-chloro-4,4-di-(4-fluorophenyl)butane. After the addition is complete, the whole is stirred and refluxed for 65 hours. After cooling the reaction mixture, there are added 70 parts water. The organic layer is separated, dried over potassium carbonate, filtered and evaporated. The solid residue is triturated in diisopropyl-ether, filtered off again and recrystallized from a mixture of 120 parts acetone and 80 parts 4methyl-2-pentanone, yielding the crude product. After recrystallization of this crop from 80 parts acetone, 1-[4,4-di-(4fluorophenyl)-butyl]-4-(2-oxo-1benzimidazolinyl)-piperidine is obtained, melting point 217°C to 219°C. | [Brand name]
Orap (Teva). | [Therapeutic Function]
Antipsychotic | [General Description]
Pimozide, 1-[1-[4,4-bis(p-fluorophenyl)butyl]-4-piperidyl]-2-benzimidazolinone (Orap), isa white to creamy white solid (pKa=9.42). Pimozide is 50%absorbed after oral administration. It is metabolized byCYP450 enzymes, in particular the CYP3A4 and CYP1A2isozymes, to inactive metabolites. Pimozide is excreted in theurine and to a lesser extent in the feces. Toxic effects may beproduced with pimozide in the presence of inducers or inhibitorsof CYP3A4 and CYP1A2. Pimozide is also a stronginhibitor of CYP2D6 without appearing to be an importantsubstrate of this isoform.99 The use of pimozide in the UnitedStates is small, but it is a critical drug for many patients withGilles de la Tourette disorder who cannot tolerate haloperidol. | [Biological Activity]
Dopamine D 2 -receptor antagonist and antipsychotic which binds with very high affinity to the cloned rat 5-HT 7 receptor (K i = 0.5 nM). | [Biochem/physiol Actions]
D2 dopamine receptor antagonist; binds with high affinity to the cloned 5-HT7 receptor; Ca2+ channel antagonist; antipsychotic | [Pharmacology]
Pimozide is an orally active antipsychotic drug product which shares with other antipsychotics the ability to blockade dopaminergic receptors on neurons in the central nervous system. However, receptor blockade is often accompanied by a series of secondary alterations in central dopamine metabolism and function which may contribute to both pimozide's therapeutic and untoward effects. In addition, pimozide, in common with other antipsychotic drugs, has various effects on other central nervous system receptor systems which are not fully characterized. Pimozide also has less potential for inducing sedation and hypotension as it has more specific dopamine receptor blocking activity than other neuroleptic agents (and is therefore a suitable alternative to haloperidol). | [Clinical Use]
Antipsychotic | [Synthesis]
Pimozide, 1-[1-[4,4-bis-(p-fluorophenyl)butyl]-4-piperidyl]-2-benzymidazoli�none (6.6.5), is structurally very similar to droperidol with the exception of the presence of a double bond in the piperidine ring and the substitution of a p-fluorobutyrophenone group on the nitrogen atom of the piperidine ring with a 4,4-bis-(p-fluorophenyl)-butyl radical. 4,4-bis-(p-fluorophenyl)-butylchloride (bromide) (6.6.3), which is needed for the synthesis of pimozide as well as fluspirylene and penfluridol, is synthesized by reacting of two moles of 4-p-fluorophenylmagnesiumbromide with cyclopropancarboxylic acid ester, which results in the formation of bis-(4-p-fluorophenyl)cyclopropylcarbinol (6.6.1). Treatment of this with thionyl chloride (phosphorous tribromide) leads to open�ing of the cyclopropyl ring, forming 1,1-bis-(4-fluorophenyl)-4-chloro(bromo)-1- butene (6.6.2). Reduction of the double bond using hydrogen over a palladium catalyst leads to the formation of 1,1-bis-(4-fluoro- phenyl)butyl chloride (bromide) (6.6.3) [60–63].
In terms of pharmacological action, pimozide is similar to haloperidol. It is used in hospi�tals as well as in outpatient settings for supportive therapy of patients suffering from schiz�ophrenia, paranoid conditions, and mental and neurotic disorders with paranoid characteristics. It is unfit for use in severe psychoses because it does not possess psychomotor-sedative action. It is used for treating patients who suffer from Turretts’s syn�drome. 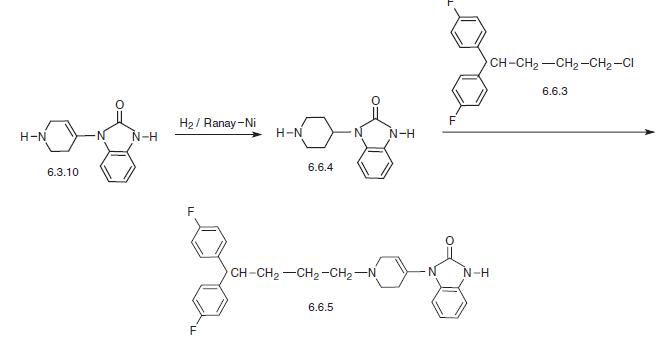
| [in vitro]
pimozide displayed high affinity for dopamine receptors. the ki values for d2, d3, and d4 were 2.4, 0.2, and 1.8 nm, respectively [3]. | [in vivo]
in hungry rats, pimozide attenuated lever-pressing and running for food reward. pimozide pretreatment attenuated acquisition of a lever-pressing habit motivated by food reward in a dose-dependent manner[4]. in 31 male wistar rats self-administering cocaine, pimozide caused a dose-dependent (0.0625–0.5 mg/kg) acceleration of responding [5]. | [Drug interactions]
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: increased risk of convulsions with
tramadol; enhanced hypotensive and sedative
effects with opioids; increased risk of ventricular
arrhythmias with methadone.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with anti-arrhythmics that prolong
the QT interval - avoid with amiodarone and
disopyramide (risk of ventricular arrhythmias).
Antibacterials: avoid with macrolides and moxifloxacin
(increased risk of ventricular arrhythmias).
Antidepressants: concentration increased by SSRIs
- avoid; increased risk of ventricular arrhythmias
with SSRIs and tricyclics - avoid; increased risk of
ventricular arrhythmias with delamanid.
Antiepileptics: antagonises anticonvulsant effect.
Antifungals: avoid with imidazoles and triazoles -
risk of ventricular arrhythmias.
Antimalarials: avoid with artemether/lumefantrine and
piperaquine with artenimol; increased risk of ventricular
arrhythmias with mefloquine and quinine - avoid.
Antipsychotics: increased risk of ventricular
arrhythmias with droperidol, phenothiazines,
risperidone or sulpiride - avoid; concentration
possibly increased by lurasidone.
Antivirals: concentration increased by atazanavir,
boceprevir, efavirenz, fosamprenavir, indinavir,
ritonavir, saquinavir and telaprevir, increased risk of
ventricular arrhythmias - avoid.
Anxiolytics and hypnotics: increased sedative effects.
Aprepitant: avoid concomitant use.
Atomoxetine: increased risk of ventricular arrhythmias.
Beta-blockers: increased risk of ventricular
arrhythmias with sotalol.
Cobicistat: concentration possibly increased by
cobicistat - avoid.
Cytotoxics: use crizotinib with caution; avoid with
lapatinib and idelalisib; increased risk of ventricular
arrhythmias with panobinostat and vandetanib -
avoid; increased risk of ventricular arrhythmias with
arsenic trioxide.
Diuretics: increased risk of ventricular arrhythmias
due to hypokalaemia.
Fosaprepitant: avoid concomitant use.
Grapefruit juice: avoid concomitant use.
Ivabradine: increased risk of ventricular arrhythmias. | [Metabolism]
Pimozide is metabolised in the liver via the cytochrome P450
isoenzyme CYP3A4 and to a lesser extent by CYP2D6
mainly by N-dealkylation and excreted in the urine and
faeces in the form of metabolites and unchanged drug. | [storage]
Store at RT | [References]
[1] janssen p a, niemegeers c j, schellekens k h, et al. pimozide, a chemically novel, highly potent and orally long-acting neuroleptic drug. i. the comparative pharmacology of pimozide, haloperidol, and chlorpromazine[j]. arzneimittel-forschung, 1968, 18(3): 261-279.
[2] beaulieu j m, gainetdinov r r. the physiology, signaling, and pharmacology of dopamine receptors[j]. pharmacological reviews, 2011, 63(1): 182-217.
[3] burstein e s, ma j, wong s, et al. intrinsic efficacy of antipsychotics at human d2, d3, and d4 dopamine receptors: identification of the clozapine metabolite n-desmethylclozapine as a d2/d3 partial agonist[j]. journal of pharmacology and experimental therapeutics, 2005, 315(3): 1278-1287.
[4] wise r a, schwartz h v. pimozide attenuates acquisition of lever-pressing for food in rats[j]. pharmacology biochemistry and behavior, 1981, 15(4): 655-656.
[5] de wit h, wise r a. blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine[j]. canadian journal of psychology/revue canadienne de psychologie, 1977, 31(4): 195.
[6] opler l a, feinberg s s. the role of pimozide in clinical psychiatry: a review[j]. journal of clinical psychiatry, 1991. |
|
|