| Identification | More | [Name]
O-(4-NITROBENZYL)HYDROXYLAMINE HYDROCHLORIDE | [CAS]
2086-26-2 | [Synonyms]
1-[(AMINOOXY)METHYL]-4-NITROBENZENE HYDROCHLORIDE
1-[(AMMONIOOXY)METHYL]-4-NITROBENZENE CHLORIDE
4-NITROBENZYLOXYAMINE HYDROCHLORIDE
O-(4-NITROBENZYL)HYDROXYLAMINE HYDROCHLORIDE
O-(4-NITROBENZYL)-HYDROXYLAMMONIUM CHLORIDE
PNBA
P-NITROBENZYLOXYAMINE HYDROCHLORIDE
TIMTEC-BB SBB003507
4-nitrobenzyloxyamine
o-(p-nitrophenylmethyl)-hydroxylaminmonohydrochloride
o-[(4-nitrophenyl)methyl]-hydroxylaminmonohydrochloride
O-(4-nitrobenzyl)hydroxylammonium hydrochloride
O-(4-Nitrobenzyl)hydroxylamineHCl
O-4-Nitrobenzylhydroxylamine Hydrochloride [for HPLC Labeling] | [EINECS(EC#)]
218-228-6 | [Molecular Formula]
C7H9ClN2O3 | [MDL Number]
MFCD00012954 | [Molecular Weight]
204.61 | [MOL File]
2086-26-2.mol |
| Questions And Answer(Q&A) | Back Directory | [Preparation]
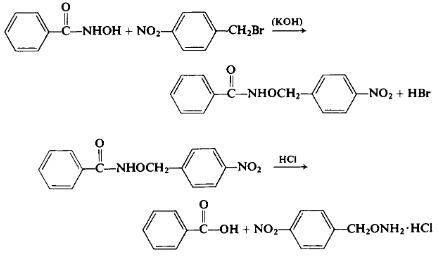
To a solution of 25 gm (0.183 mole) of benzohydroxamic acid in 28 ml of ethanol and 10.2 gm (0.184 mole) of potassium hydroxide in 40 ml of water is added a hot solution of 39.4 gm (0.184 mole) of p-nitrobenzyl bromide in ethanol. The mixture is then heated under a reflux condenser for 45 min and cooled. The precipitated crystals are separated and crystallized from ethanol. (No yield reported.) A solution of 15 gm (0.055 mole) of the above o-p-nitrobenzylbenzohy-droxamic acid (p-nitrobenzyl benzohydroxamate) in 125 ml of hot ethanol is heated to reflux for 25 min with 150 ml of cone, hydrochloric acid. To prevent the precipitation of the final product during the work-up, the following steps are carried out rapidly. The clear solution is diluted with 150 ml of water and while still warm benzoic acid and other by-products are separated by extraction with 200 ml of chloroform. The aqueous layer is evaporated to dryness under reduced pressure to afford a crude yield of 10.75 gm (96%). The residue is recrystallized from hot 2 Ν hydrochloric acid and washed in turn with ethanol and with ether to afford a solid, m.p. 217°C.
The free base may be produced by neutralizing the salt with sodium carbonate solution; O-p-nitrobenzylhydroxylamine, m.p. 56°C (recrystallized from petroleum ether). By a similar technique, O-allylhydroxylamine hydrochloride, m.p. 172°C (dec.) was also prepared.
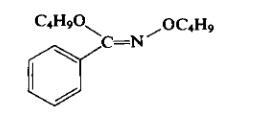
An examination of the product of butylating benzohydroxamic acid showed that a nonacidic fraction and an equal weight of an acidic fraction were readily isolated. The nonacidic fraction contained mainly Ο, Ο '-dibutyl benzohydroxi-mate (b.p. 175-180°C/13 mm Hg) and some aniline (probably formed during the reaction by a Lossen rearrangement). On hydrolysis with eth-anolic hydrochloric acid, the hydroximate produced ethyl benzoate and O-butylhydroxylamine hydrobromide (m.p. 159-161°C; hydrochloride, m.p. 156-157°C).
The acidic fraction contained mainly O-butylbenzohydroxamic acid (butyl benzohydroxamate) which on hydrolysis formed O-butylhydroxylamine hydrochloride and benzoic acid. It has been contended that in this particular work, the presence of an Ν,Ο-dibutylbenzohy-droxamic acid had been overlooked. The major source of O-butylhydroxylamine hydrochloride is from the acidic fraction, although the yield is not particularly high. The initial preparation of the butylated benzohydroxamic acid derivatives has been repeated recently. The analysis of the reaction products by vapor-phase chromatography showed it to contain 43% of O-butylbenzohydroxamic acid (butyl benzohydroxamate), 17% of iV,0-dibutyl-benzohydroxamic acid, and 8% of n-butyl(Z)-0-n-butylbenzohydroximate:
The nature of the remaining 32% of the crude reaction product was not determined. The use of benzohydroxamic acid has been extended to the preparation of a variety of O-substituted hydroxylamines. For example, halocarboxylic acids have been used as alkylating agents to produce α-aminooxy acids of the type, NH20(CH2)„C02H.
A variety of O-benzylhydroxylamines, O-aralkylhydroxylamines, as well as simpler O-substituted hydroxylamines have also been prepared. In some of these preparations, the hydrolysis of the O-substituted benzohydroxamic acid was carried out with hot alcoholic hydrogen chloride. This treatment evidently prevented the O-N cleavage which had been observed during the aqueous hydrolysis of the substituted benzohydroxamic acids. |
| Safety Data | Back Directory | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [RTECS ]
NC4400050
| [F ]
3-8-9 | [TSCA ]
Yes | [HazardClass ]
IRRITANT | [HS Code ]
29280000 | [Storage Class]
11 - Combustible Solids |
| Hazard Information | Back Directory | [Chemical Properties]
LIGHT YELLOW POWDER | [Uses]
A strong UV chromophore used in derivatization of reducing sugars for ultraviolet absorption detection in HPLC analyses. | [Uses]
As a strong UV chromophore, O-(4-Nitrobenzyl)hydroxylamine hydrochloride can be used in derivatization of reducing sugars for ultraviolet absorption detection in HPLC analyses.
| [Synthesis]
General procedure for the synthesis of 4-nitrobenzylhydroxylamine hydrochloride from 2-((4-nitrobenzyl)oxy)isoindoline-1,3-dione: Compound 18 (2.00 g, 6.71 mmol) was suspended in EtOH (10 mL), and concentrated aqueous hydrochloric acid (20 mL) was slowly added under magnetic stirring. The reaction mixture was heated to reflux for 3 hours. After completion of the reaction, it was slightly cooled and H2O (30 mL) was added, followed by washing with CHCl3 (20 mL × 1). The aqueous phase was separated and concentrated under reduced pressure to afford 4-nitrobenzylhydroxylamine hydrochloride 19 (1.37 g, quantitative yield) as a milky white solid with melting point 179.1-205.9 °C (literature value 217 °C).1H NMR (300 MHz, DMSO-d6) δ 11.37 (br s, 2H), 8.26 (d, J=8.6 Hz, 2H), 7.68 (d , J=8.6 Hz, 2H), 5.22 (s, 2H). | [References]
[1] Patent: WO2008/124878, 2008, A1. Location in patent: Page/Page column 42
[2] Patent: US2014/378399, 2014, A1. Location in patent: Paragraph 0122
[3] MedChemComm, 2013, vol. 4, # 8, p. 1156 - 1165
[4] European Journal of Medicinal Chemistry, 2016, vol. 108, p. 564 - 576
[5] Arkivoc, 2018, vol. 2018, # 4, p. 139 - 148 |
|
|