| Identification | Back Directory | [Name]
Fosamprenavir calcium | [CAS]
226700-81-8 | [Synonyms]
Lexiva
Telzir
GW-433908G
FOSAMPRENAVIR
fosamprenavir calcium
FOSAMPRENAVIR, CALCIUM SALT
Fosamprenavir & Fosamprenavir Calcium
calcium [(3s)-oxolan-3-yl] n-[(2s,3r)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-1-phenyl-3-phosphonatooxy-butan-2-yl]carbamate
[(2R,3S)-1-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-[[(3S)-oxolan-3-yl]oxycarbonylamino]-4-phenyl-butan-2-yl]oxyphosphonic acid | [EINECS(EC#)]
607-123-4 | [Molecular Formula]
C25H34CaN3O9PS | [MDL Number]
MFCD08141843 | [MOL File]
226700-81-8.mol | [Molecular Weight]
623.669 |
| Chemical Properties | Back Directory | [Appearance]
White Microcrystalline Needles | [Melting point ]
282-2840C | [storage temp. ]
-20°C Freezer, Under Inert Atmosphere | [solubility ]
Aqueous Acid (Slightly), DMF (Slightly) | [form ]
Solid | [color ]
White to Off-White | [BCS Class]
2 | [InChIKey]
PMDQGYMGQKTCSX-HQROKSDRSA-L | [SMILES]
[O-]P(O[C@H](CN(CC(C)C)S(C1=CC=C(N)C=C1)(=O)=O)[C@@H](NC(O[C@@H]2COCC2)=O)CC3=CC=CC=C3)([O-])=O.[Ca+2] |
| Hazard Information | Back Directory | [Chemical Properties]
White Microcrystalline Needles | [Uses]
HIV protease inhibitor; water soluble prodrug of amprenavir | [Uses]
Protease inhibitor, anti-HIV agent | [Description]
Fosamprenavir (calcium salt) is an orally bioavailable prodrug of the HIV-1 protease inhibitor amprenavir (Item No. 15369).1 Fosamprenavir has improved solubility compared with amprenavir, and its pharmacokinetics, either during fasting or with a low- or high-fat meal, suggest that it could be effective using fewer tablets and a less complex dosing schedule than other HIV treatments.2 Formulations containing fosamprenavir are used for adult and pediatric patients with HIV infection, especially as an initial antiretroviral therapy.3 | [Description]
Fosamprenavir, a prodrug of the HIV protease inhibitor amprenavir, is indicated for the
oral treatment of HIV infection in adults in combination with other antiretroviral agents.
Although amprenavir has excellent antiviral potency and good tolerability, its watersolubility
is poor (0.04 mg/ml). As a result, the formulation of the agent includes a high
percentage of organic excipients to facilitate gastric dissolution, which limits the amount
of active drug that can be formulated per capsule. Fosamprenavir is a highly soluble
phosphate ester of amprenavir. It allows more convenient dosing and reduction in pill
counts as compared to amprenavir. Fosamprenavir is readily prepared in two steps
starting from a key intermediate used in the synthesis of amprenavir, by phosphorylating
a hydroxyl group and subsequently reducing a p-nitrophenyl to a p-aminophenyl group.
Fosamprenavir has little or no antiviral activity in vitro. After oral administration, it is
rapidly and almost completely hydrolyzed by phosphatases in the gut epithelium to
amprenavir prior to reaching systemic circulation. The time to reach peak plasma
concentration of amprenavir is approximately 2.5 h and the plasma elimination half-life
is approximately 7.7 h. Amprenavir is metabolized in the liver by CYP3A4 and >90% of
the dose is excreted as metabolites in urine and feces. In most patients, fosamprenavir is
administered at daily doses of 700–1400 mg in conjunction with ritonavir. Monotherapy
with fosamprenavir is only recommended in antiretroviral therapy-na?ve patients and the
dosing regimen is 1400 mg twice daily. The most common adverse events experienced
with fosamprenavir are diarrhea, nausea, vomiting, headache and rash. | [Originator]
Vertex (US) | [Brand name]
Lexiva (GlaxoSmithKline). | [General Description]
Fosamprenavir is used in combination with otherHIV drugs in adult patients. Like the other PIs, this compoundis a prodrug that produces the active drug uponhydrolysis. In this case, the active drug is amprenavir, apeptidomimetic transition state inhibitor. Fosamprenaviris typically administered in combination with RTinhibitors. | [Clinical Use]
Fosamprenavir calcium has been approved for the treatment of HIV in adults when used in combination
with other anti-HIV drugs. It is a prodrug that, on hydrolysis by serum phosphatases, gives rise to
amprenavir, which is a peptidomimetic transition-state inhibitor that targets HIV-1 protease and reduces
the viral replication and, thus, the infectiousness of HIV-1. It is commonly administered in combination
with RT inhibitors to produce excellent efficacy in patients with AIDS. The drug is administered as two
700 mg tablets twice daily or, in combination with ritonavir, can be given as two 700 mg tables once daily
or one 700 mg tablet twice daily. As a result, formaprenavir lowers the "pill burden" in patients with AIDS. | [Synthesis]
The synthesis of
fosamprenavir (X) started with a known amino alcohol 91. N,N-Dibenzyl-L-phenylalaninal (87) was prepared
by reduction of L-phenylalanine (86) to L-phenylalaninol
followed by N,N-dibenzylation and oxidation to the
aldehyde 87 using pyridine-sulfur trioxide complex at room
temperature. A large excess of lithium shot was stirred in a
solution of aldehyde 87 and bromochloromethane in THF at
-65??C. The reaction mixture was subsequently allowed to
warm up to room temperature to provide the diastereomeric
epoxide mixture (6:1) which was quenched with 6N aqueous
HCl and set standing overnight to provide the salt
precipitate. Recrystallization from methanol gave optically
pure dibenzylaminochlorohydrin hydrochloride (88) in 38-
45% yield. Hydrogenolysis under standard conditions gave
deprotected aminochlorohydrin hydrochloride 89 as a
crystalline white solid. Conversion to desired N-Bocepoxide
90 was accomplished by the introduction of the Boc
group followed by cyclization. N-Boc-epoxide 90 was
then converted to amino alcohol 91 by refluxing with isobutylamine
in EtOH. Treatment of the amino alcohol91 with p-nitrobenzene sulphonyl chloride in toluene at
80??C followed by acid hydrolysis of the Boc group
furnished sulphonamide 93 in 73% yield. The carbamate 95
was prepared by refluxing 93 with (S)-tetrahydrofuryl
imidazole carboxylate (94) in EtOAc. Treatment of the
sulphonamide 95 with POCl3 followed by aqueous HCl
hydrolysis provided the phosphate intermediate, which was
then reduced by hydrogenation and converted to
fosamprenavir calcium salt X in a one-pot process in 92%
yield.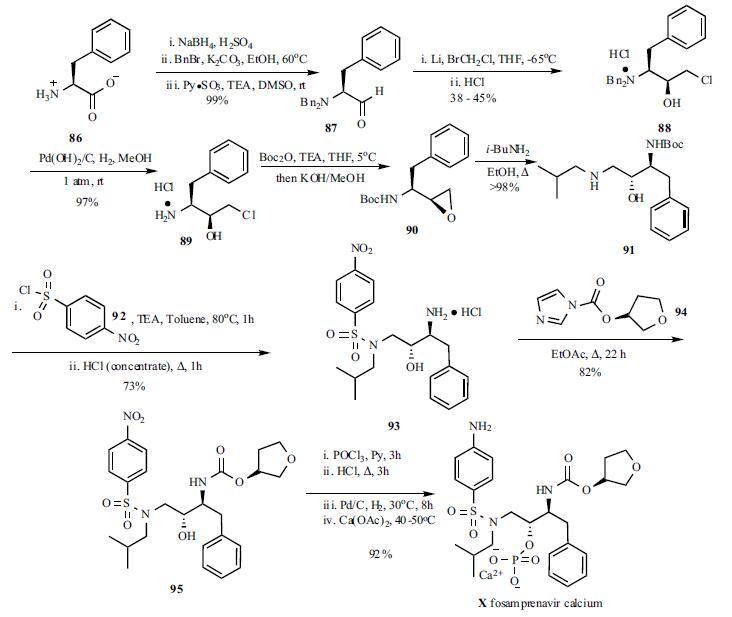
| [Drug interactions]
Potentially hazardous interactions with other drugs
Anti-arrhythmics: possibly increased concentration
of amiodarone, flecainide, lidocaine and propafenone
(increased risk of ventricular arrhythmias) - avoid.
Antibacterials: increases concentration of rifabutin
- reduce rifabutin dose; concentration significantly
reduced by rifampicin - avoid; avoid with
telithromycin in severe renal and hepatic impairment.
Anticoagulants: avoid with apixaban and
rivaroxaban.
Antidepressants: concentration reduced by St John’s
wort - avoid.
Antimalarials: use artemether/lumefantrine with
caution; possibly increases quinine concentration.
Antipsychotics: possibly inhibits aripiprazole
metabolism - reduce aripiprazole dose; possibly
increases quetiapine concentration - avoid; possibly
increases pimozide concentration (increased risk of
ventricular arrhythmias) - avoid.
Antivirals: avoid with boceprevir, raltegravir and
telaprevir; concentration of dolutegravir reduced;
concentration increased by etravirine, consider
reducing fosamprenavir dose; concentration
reduced by lopinavir, maraviroc and tipranavir,
effect on lopinavir unpredictable - avoid, avoid
with maraviroc; concentration possibly reduced by
nevirapine; avoid with raltegravir.
Anxiolytics and hypnotics: increased risk of
prolonged sedation and respiratory depression with
midazolam - avoid with oral midazolam.
Avanafil: concentration of avanafil possibly increased.
Cytotoxics: possibly increases concentration of
bosutinib and ibrutinib, avoid or consider reducing
bosutinib and ibrutinib dose.
Ergot alkaloids: increased risk of ergotism - avoid.
Immunosuppressants: monitor ciclosporin,
tacrolimus and sirolimus levels.
Lomitapide: avoid concomitant use.
Orlistat: absorption possibly reduced by orlistat.
Ranolazine: possibly increases ranolazine
concentration - avoid.
Statins: possibly increased risk of myopathy with
atorvastatin; possibly increased myopathy with
simvastatin and rosuvastatin - avoid. | [Metabolism]
Fosamprenavir is rapidly and almost completely
hydrolysed to amprenavir and inorganic phosphate as it
is absorbed through the gut epithelium, following oral
administration. The primary route of metabolism of
amprenavir is via the cytochrome P450 3A4 enzyme.
The primary route of elimination of amprenavir is
via hepatic metabolism with less than 1% excreted
unchanged in the urine and no detectable amprenavir
in faeces. Metabolites account for approximately 14%
of the administered amprenavir dose in the urine, and
approximately 75% in the faeces. | [References]
[1]. wire, m.b., et al., pharmacokinetics and safety of gw433908 and ritonavir, with and without efavirenz, in healthy volunteers. aids, 2004. 18(6): p. 897-907.
[2]. hamada, y., et al., high incidence of renal stones among hiv-infected patients on ritonavir-boosted atazanavir than in those receiving other protease inhibitor-containing antiretroviral therapy. clin infect dis, 2012. 55(9): p. 1262-9.
[3]. zheng, y., et al., antiretroviral therapy and efficacy after virologic failure on first-line boosted protease inhibitor regimens. clin infect dis, 2014. 59(6): p. 888-96.
[4]. falcoz, c., et al., pharmacokinetics of gw433908, a prodrug of amprenavir, in healthy male volunteers. j clin pharmacol, 2002. 42(8): p. 887-98. |
|
|