| Identification | Back Directory | [Name]
Obeticholic Acid | [CAS]
459789-99-2 | [Synonyms]
9470
C15636
6-Ecdca
Ocaliva
DSP-1747
6-Ethyl-cdca
Obeticholic Acid
Obetichlolic Acid
6-Ethylchenodeoxycholic acid
6alpha-Ethyl-chenodeoxycholic acid
6-Ethylchenodeoxycholic acid(Obeticholic Acid)
(3α,5β,6α,7α)-6-ethyl-3,7-dihydroxy-cholan-24-oic acid
6alpha-ethyl-chenodeoxycholic acid, 6-ECDCA, INT-747
(R)-4-((3R,5S,6R,7R,8S,9S,10S,13R,14S,17R)-6-ethyl-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoic acid
(4R)-4-[(3R,5S,6R,7R,8S,9S,10S,13R,14S,17R)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid | [EINECS(EC#)]
810-245-2 | [Molecular Formula]
C26H44O4 | [MDL Number]
MFCD16621104 | [MOL File]
459789-99-2.mol | [Molecular Weight]
420.63 |
| Chemical Properties | Back Directory | [Melting point ]
108-110 °C | [Boiling point ]
562.9±25.0 °C(Predicted) | [density ]
1.091 | [storage temp. ]
-20°C | [solubility ]
Soluble in DMSO (up to 35 mg/ml) or in Ethanol (up to 25 mg/ml) | [form ]
White solid. | [pka]
4.76±0.10(Predicted) | [color ]
White | [Optical Rotation]
[α]/D +4 to +6°, c =1.0 in methanol | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 2 months. | [InChIKey]
ZXERDUOLZKYMJM-IMGBAYNYNA-N | [SMILES]
C[C@]12CC[C@@H](O)C[C@@]1([H])[C@@H](CC)[C@@H](O)[C@@]1([H])[C@]3([H])CC[C@]([H])([C@H](C)CCC(=O)O)[C@@]3(C)CC[C@]21[H] |&1:1,4,7,9,12,14,16,20,22,29,33,r| |
| Hazard Information | Back Directory | [Definition]
ChEBI: A dihydroxy-5beta-cholanic acid that is chenodeoxycholic acid carrying an additional ethyl substituent at the 6alpha-position. A semi-synthetic bile acid which acts as a farnesoid X receptor agonist and is used for treatme
t of primary biliary cholangitis. | [Uses]
6-Ethylchenodeoxycholic Acid is a derivative of the bile acid Chenodeoxycholic Acid (C291900). 6-Ethylchenodeoxycholic Acid is a potent activator of the farnesoid X nuclear receptor which reduces liver fat and fibrosis in animal models of fatty liver disease. | [Synthesis]
The synthesis of obeticholic acid was initiated from
commercial 3|á-hydroxy-7-keto-5|?-cholan-24-oic acid (138). Fischer esterification of 138
provided methyl ester 139, which was treated with
trimethylsilyl chloride and triethylamine to protect the
secondary alcohol. Reaction of the protected alcohol with
lithium diisopropylamine and trimethylsilyl chloride gave silyl
enol ether 140. Aldol condensation with acetaldehyde and
boron trifluoride etherate followed by saponification of the
methyl ester produced enone 141. Hydrogenation of the olefin
followed by heating to reflux to epimerize the resulting ethyl
group produced the |á-ethyl ketone 142 in 62% yield from
compound 138. Reduction of the ketone in 142 with sodium
borohydride and subsequent crystallization from phosphoric
acid and water gave obeticholic acid (XIV) in 90% yield.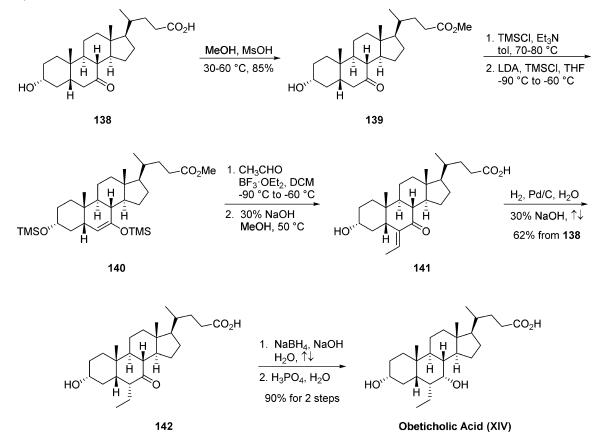
| [Enzyme inhibitor]
This semisynthetic bile acid analogue (FW = 420.63 g/mol; CAS 459789- 99-2), also named INT-747, 6α-ethyl-chenodeoxycholate, and (3α,5β,6α, 7α)-6-ethyl-3,7-dihydroxycholan-24-oic acid, is an analogue of the naturally occurring bile acid (FW = 392.57 g/mol; CAS 474-25-9). The latter is synthesized in the liver, where it conjugated to form taurochenodeoxycholate and glycol-chenodeoxycholate, reducing its pKa and increasing retention in the gastrointestinal tract, until reabsorption by the ileum. Chenodeoxycholate is the most active physiological ligand known for the farnesoid X receptor, or FXR (encoded by the NR1H4 gene in humans) that translocates to the nucleus, dimerizes, and binds to hormone response elements. Obeticholic acid reduces bacterial translocation and invasion in cirrhotic rats by restoring intestinal barrier integrity (through increased expression of tight junction proteins) and by inhibiting inflammation. Obeticholate likewise up-regulated expression of the FXR-associated gene small heterodimer partner (SHP). | [storage]
+4°C |
| Questions And Answer | Back Directory | [Description]
Obeticholic acid is a semi-synthetic bile acid analogue and acts as a farnesoid-X receptor (FXR) agonist. It is used for the treatment of primary biliary cholangitis. It is also under investigation for the treatment of other liver diseases,primary biliary cirrhosis, bile acid diarrhea and related disorders. Study has shown that it also has potential for treating nonalcoholicsteatohepatitis (NASH), and portal hypertension. Obeticholic acid takes effect through acting s the agonist of the farnesoid X receptor (FXR), which is the regulator of bile and cholesterol metabolism in the liver.
| [Indications and Usage]
Obeticholic acid is also called 6-Ethylchenodeoxycholic acid. It is a new derivative of chenodeoxycholic acid (CDCA) in human primary bile acids, a natural ligand for farnesoid x receptors (FXR). Obeticholic acid was developed by American pharmaceutical company Intercept as the first drug to treat cholestatic liver disease in 20 years, and it is administered on patients that do not respond well to or cannot tolerate the old standard treatment drug ursodeoxycholic acid. Obeticholic acid has also been tested to treat a more common form of fatty liver – non-alcoholic fatty liver disease (NAFLD). Obeticholic acid can also be developed to treat other liver and intestine diseases.
| [Mechanisms of Action]
Obeticholic acid belongs to FXR stimulants, activating FXRs and indirectly inhibiting Cytochrome P450 Family 7 Subfamily A Member 1 (CYP7A1) expression. As CYP7A1 is a rate-limiting enzyme of bile acid biosynthesis, obeticholic acid can inhibit the bile acid synthesis and is used to treat primary biliary cirrhosis.
| [Clinical Research]
In a placebo control phase III clinical trial, Obeticholic acid increased levels of two biomarkers indicating lowered risk in liver transplant. The composite end point of the clinical research is that alkaline phosphatase lowered by at least 15%, serum alkaline phosphatase activity was 1.67 times lower than the normal upper limit, and bilirubin levels were within normal range; alkaline phosphatase is a biomarker indicating liver disease severity. An American 6-week, multi-center, randomized, and double-blind clinical trial included 64 cases of type 2 diabetes patients with NAFLD, and it proved that Obeticholic acid not only increased insulin sensitivity, but also improved liver inflammation and fibrosis levels, and it has certain weight-reducing effects. However, this conclusion requires further investigation with a larger and more long-term follow-up, as well as scientific backing in liver pathology.
| [References]
Verbeke, L, et al. "Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats."Hepatology 59.6(2014):2286-98.
Silveira, M. G., and K. D. Lindor. "Obeticholic acid and budesonide for the treatment of primary biliary cirrhosis." Expert Opinion on Pharmacotherapy 15.3(2014):365.
|
|
|