| Identification | Back Directory | [Name]
Naloxone | [CAS]
465-65-6 | [Synonyms]
C07252
NALOXONE
NSC 70413
l-naloxone
-bcd)furanone
Naloxone Base
,5-bcd)furanone
Naloxone solution
Naloxone impurity
1803153937@189.cn
Naloxone (125 mg)
-bcd]furan-5(6h)-one
n-allyl-noroxymorphone
Naloxone, (controlled)
Nafoxone Hydrochloride
naloxone ada@tuskwei.com sky
Methanol (test Naloxone, 1.0 mg/mL)
l-n-allyl-14-hydroxynordihydromorphinone
1-n-allyl-14-hydroxynordihydromorphinone
9: PN: WO03037310 FIGURE: 4 claimed sequence
1-n-allyl-7,8-dihydro-14-hydroxynormorphinone
l-n-allyl-7,8-dihydro-14-hydroxynormorphinone
17-Allyl-4,5α-epoxy-3,14-dihydroxymorphinan-6-one
Normorphinone, N-allyl-7,8-dihydro-14-hydroxy- (7CI)
17-allyl-4,5alpha-epoxy-3,14-dihydroxymorphinan-6-one
17-allyl-4,5alpha-epoxy-3,14-dihydroxy-morphinan-6-on
17-allyl-4,5-alpha-epoxy-3,14-dihydroxymorphinan-6-one
Morphinan-6-one, 17-allyl-4,5a-epoxy-3,14-dihydroxy- (8CI)
4,5-alpha-epoxy-3,14-dihydroxy-17-(2-propenyl)-morphinan-6-on
5-epoxy-3,14-dihydroxy-17-(2-propenyl)-(5alpha)-morphinan-6-on
(5α)-4,5-Epoxy-3,14-dihydroxy-17-(2-propen-1-yl)morphinan-6-one
(5alpha)-3,14-Dihydroxy-17-prop-2-en-1-yl-4,5-epoxymorphinan-6-one
Morphinan-6-one,4,5-epoxy-3,14-dihydroxy-17-(2-propen-1-yl)-, (5a)-
Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-(2-propenyl)-, (5a)- (9CI)
12-allyl-7,7a,8,9-tetrahydro-3,7a-dihydroxy-4ah-8,9c-iminoethanophenanthro(4
12-allyl-7,7a,8,9-tetrahydro-3,7a-dihydroxy-4ah-8,9c-iminoethanophenanthro(4,5
12-allyl-7,7a,8,9-tetrahydro-3,7a-dihydroxy-4ah-8,9c-iminoethanophenanthro[4,5
12-Allyl-7,7a,8,9-tetrahydro-3,7a-dihydroxy-4aH-8,9c-iminoethanophenanthro[4,5-bcd]furan-5(6H)-one
(4R)-6,10aβ-Dihydroxy-11-allyl-4α,5-epoxy-10β,4aβ-(epiminoethano)-1,2,3,4,4a,9,10,10a-octahydrophenanthrene-3-one | [EINECS(EC#)]
207-365-7 | [Molecular Formula]
C19H21NO4 | [MDL Number]
MFCD00242634 | [MOL File]
465-65-6.mol | [Molecular Weight]
327.37 |
| Chemical Properties | Back Directory | [Melting point ]
184° (Lewenstein), 177-178° (Sankyo Co.) | [alpha ]
D20 -194.5° (c = 0.93 in CHCl3) | [Boiling point ]
465.27°C (rough estimate) | [density ]
1.2223 (rough estimate) | [refractive index ]
1.5000 (estimate) | [Fp ]
9℃ | [storage temp. ]
2-8°C | [solubility ]
Chloroform (Slightly, Heated, Sonicated), DMSO (Slightly), Methanol (Slightly), | [form ]
Solid | [pka]
pKa 7.94/7.82(H2O,t =20/37,I<0.01) (Uncertain) | [color ]
White to Off-White | [InChI]
1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | [InChIKey]
UZHSEJADLWPNLE-GRGSLBFTSA-N | [SMILES]
Oc1ccc2C[C@H]3N(CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O)CC=C | [EPA Substance Registry System]
Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-( 2-propenyl)-, (5.alpha.)-(465-65-6) |
| Hazard Information | Back Directory | [Uses]
antineoplastic | [Definition]
ChEBI: A synthetic morphinane alkaloid that is morphinone in which the enone double bond has been reduced to a single bond, the hydrogen at position 14 has been replaced by a hydroxy group, and the methyl group attached to the nitrogen has been replaced by an all
l group. A specific opioid antagonist, it is used (commonly as its hydrochloride salt) to reverse the effects of opioids, both following their use of opioids during surgery and in cases of known or suspected opioid overdose. | [Description]
It is worth mentioning that N-allylic substitution in a number of morphine derivatives, as
a rule, leads to antagonistic properties. Naloxone is a few times stronger than nalorphine
as an antagonist. It blocks opiate receptors. It eliminates central and peripheral action of
opioids, including respiratory depression. Naloxone is used upon overdose of narcotic
analgesics. | [Originator]
Narcan,Du Pont,US,1971 | [Manufacturing Process]
10 grams of 14-hydroxydihydromorphinone (oxymorphone) was converted
into its diacetate by warming it on the steam bath with 80 cc of acetic
anhydride for about 2 hours. The acetic anhydride was removed on the water
bath under a vacuum of about 30 mm absolute pressure. The melting point of
the residue was 220°C. The residue was taken up in 100 cc of chloroform. An
equal amount by weight of cyanogen bromide was added and the mixture was
refluxed at about 60°C for about 5 hours. After refluxing, the mixture was
washed with 100 cc of a 5% aqueous hydrochloric acid solution, dried over
sodium sulfate and the chloroform removed by evaporation under a vacuum of
about 30 mm. The residue had a melting point of 240°C.
The residue was then heated at about 90°C for 16 hours on a steam bath with
300 cc of 20% aqueous hydrochloric acid solution, and treated with a small
amount, e.g., 1 gram of charcoal. The hydrochloric acid was then removed
under a vacuum of 15 mm, the residue dissolved in 30 cc of water and
precipitated by the addition of 2.4 cc of concentrated aqueous ammonia. The
precipitate was filtered off and dried. It consists of 14-
hydroxydihydronormorphinone. It is soluble in ethanol.
The 14-hydroxydihydronormorphinone was suspended in 200 cc of pure ethyl
alcohol, half its weight of sodium bicarbonate and half its weight of allyl
bromide added and the resulting mixture was refluxed at about 75°C for 48
hours. The solution was cooled, e.g., to 10°C and filtered and the alcohol
removed under a vacuum of 30 mm. The residue was dissolved in chloroform
and filtered. The chloroform was removed under a vacuum of 30 mm and the
residue was crystallized from ethylacetate. The crystallized product, N-allyl-
1,4-hydroxydihydronormorphinone, has a melting point of 184°C, is soluble in
chloroform and insoluble in petroleum ether. The yield amounts to 20% based
on the weight of the reacted 14-hydroxydihydromorphinone. | [Brand name]
Narcan (Bristol-Myers Squibb); Narcan (Endo). | [Therapeutic Function]
Narcotic antagonist | [Biological Functions]
Because of its fast onset (minutes), naloxone (Narcan)
administered IV is used most frequently for the reversal
of opioid overdose. However, it fails to block some
side effects of the opioids that are mediated by the δ-
receptor, such as hallucinations. The rapid offset of
naloxone makes it necessary to administer the drug repeatedly
until the opioid agonist has cleared the system
to prevent relapse into overdose. The half-life of naloxone
in plasma is 1 hour. It is rapidly metabolized via glucuronidation in the liver and cleared by the kidney.
Naloxone given orally has a large first-pass effect, which
reduces its potency significantly. Often an overshoot
will follow the administration of naloxone for overdose.
The heart rate and blood pressure of the patient may
rise significantly. The overshoot is thought to be due to
precipitation of acute withdrawal signs by naloxone.
Given alone to nonaddicts, naloxone produces no pharmacological
effects.
Naloxone is approved for use in neonates to reverse
respiratory depression induced by maternal opioid use.
In addition, naloxone has been used to improve circulation
in patients in shock, an effect related to blockade of
endogenous opioids. Other experimental and less well
documented uses for naloxone include reversal of coma
in alcohol overdose, appetite suppression, and alleviation
of dementia from schizophrenia. Side effects of
naloxone are minor. | [General Description]
Naloxone (Narcan) is a pure antagonist at allopioid receptor subtypes. Structurally, it resembles oxymorphoneexcept that the methyl group on the nitrogen isreplaced by an allyl group. This minor structural change retains high binding affinity to the receptor, but no intrinsicactivity. It is used to reverse the respiratory depressant effectsof opioid overdoses.
Naloxone is administered intravenously with an onset ofaction within 2 minutes. Because it is competing with theopioid for the receptor sites, the dose and frequency of administrationwill depend on the amount and type of narcoticbeing antagonized. Overdoses of long-acting opioids(methadone) may require multiple IV doses of naloxone orcontinuous infusions. Neonates born to opioid-exposedmothers may be given IV naloxone at birth to reverse the effectsof opiates.
Very few metabolism studies on naloxone have beenconducted, although the major metabolite found in the urineis naloxone-3-glucuronide. | [Clinical Use]
Naloxone has no analgesic activity. The compound
is the standard antidote to treat opioid adverse
reactions, opioid overdoses, or to stop an intended
use of an opioid compound. Typical indications
are inhibition of opioid-induced respiratory
depression, termination of opioid anesthesia
or protection of neonates following opioid
treatment during labor. Naloxone has a short
duration of action and repetitive administration
may be necessary to antagonize longer acting
agonists. To avoid parenteral misuse of nonscheduled
oral opioid formulations (tilidine,
pentazcocine), a small amount of naloxone is
added which is orally inactivated, but is fully
active after parenteral administration.
Naloxone is orally inactive and is only used
parenterally in single or repetitive doses of 0.4–
2 mg up to a total dose of 10 mg, as an intravenous
bolus injection or by infusion. The compound
is more potent against pure opioid agonists
than against mixed agonist – antagonists.
Caution should be used in opioid-dependent persons
or in persons under high-dose opioid treatment,
as naloxone may precipitate an acute withdrawal
reaction. Naloxone is relatively free of
side effects. Nausea, vomiting, and convulsions
have occasionally been reported. | [Synthesis]
Naloxone, (-)-17-(allyl)-4,5-epoxy-3,14-dihydroxymorphinan-6-one (3.1.92),
is synthesized by the alkylation of 14-hydroxydihydronormorphinane (3.1.82) by allylbro�mide [55¨C58].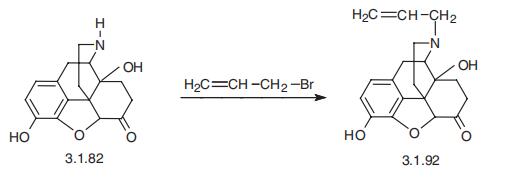
|
| Safety Data | Back Directory | [Hazard Codes ]
F,T | [Risk Statements ]
11-23/24/25-39/23/24/25 | [Safety Statements ]
7-16-36/37-45 | [RIDADR ]
UN1230 class 3 PG 2 Methanol, solution | [WGK Germany ]
3 | [HS Code ]
2939190000 | [Storage Class]
3 - Flammable liquids | [Hazard Classifications]
Acute Tox. 3 Dermal
Acute Tox. 3 Inhalation
Acute Tox. 3 Oral
Flam. Liq. 2
STOT SE 1 | [Hazardous Substances Data]
465-65-6(Hazardous Substances Data) | [Toxicity]
An opiate antagonist devoid of agonist activity except
for mild, specific effects at very high doses. Naloxone displays a
high affinity for the μ-opioid receptor, a lesser affinity for the kopioid
receptor and has some affinity for δ-opioid receptor subtypes.
Naloxone produces a rapid and profound reversal of the effects of opioid administration (e.g., 1 mg, i.v., blocks the effects
of 25 mg of heroin). Naloxone also antagonizes the analgesia
induced by placebo, acupuncture, and stress, and in animals the
hypotension due to hypovolemia or spinal cord injury. Naloxone
has a short half-life (about 1 h in plasma) and is not administered
orally because of rapid, “first-pass” metabolism. |
|
|