| Identification | Back Directory | [Name]
CARBENICILLIN | [CAS]
4697-36-3 | [Synonyms]
Pyocianil
onamic acid
CARBENICILLIN
Carboxybenzylpenicillin
CARBENICILLIN READY MADE
Carbenicillinum natricum
(α-Carboxybenzyl)penicillin
Carboxybenzylpenicillin acid
ALPHA-CARBOXYBENZYLPENICILLIN
carbenicillin ada@tuskwei.com sky
6-(α-Carboxyphenylacetamido)penicillanic acid
Carbenicillin (base and/or unspecified salts)
6-(alpha-Carboxyphenylacetamido)penicillanic acid
Malonamic acid, N-(2-carboxy-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-6-yl)-2-phenyl- (7CI, 8CI)
N-[(2S,5R,6R)-2-Carboxy-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptan-6-yl]-2-phenylmalonamidic acid
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[(carboxyphenylacetyl)amino]-3,3-dimethyl-7-oxo-, (2S,5R,6R)-
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[(carboxyphenylacetyl)amino]-3,3-dimethyl-7-oxo-, [2S-(2α,5α,6β)]- | [EINECS(EC#)]
225-171-0 | [Molecular Formula]
C17H18N2O6S | [MDL Number]
MFCD00242604 | [MOL File]
4697-36-3.mol | [Molecular Weight]
378.4 |
| Chemical Properties | Back Directory | [Boiling point ]
737.8±60.0 °C(Predicted) | [density ]
1.53±0.1 g/cm3(Predicted) | [storage temp. ]
−20°C
| [solubility ]
Soluble in DMSO | [form ]
Powder | [pka]
pKa 2.22±0.05(H2O
t = 25.0
I = 0.15 (KCl)) (Uncertain);3.25±0.02 (Uncertain) | [Water Solubility ]
H2O: 100mg/mL
ethanol: 100mg/mL | [CAS DataBase Reference]
4697-36-3 |
| Hazard Information | Back Directory | [Definition]
ChEBI: A penicillin antibiotic having a 6beta-2-carboxy-2-phenylacetamido side-chain. | [Chemical Properties]
Its sodium salt ([4800-94-6]) is a white or off-white crystalline powder that is easily soluble in water, soluble in methanol, slightly soluble in ethanol, and practically insoluble in benzene or ether. It is highly hygroscopic, thermally unstable, has a slight odor, and tastes bitter. | [Originator]
Pyopen,Beecham,Switz.,1968 | [Uses]
Antibacterial. | [Indications]
Carbenicillin has a broad spectrum of antibacterial use with respect to Gram-negative and
Gram-positive microorganisms. However, using this drug for infections caused by Gram�positive microorganisms is pointless. It is used for diseases such as urinary tract infections,
septicemia, endocarditis, meningitis, osteomelitis, peritonitis, purulent otitis, infected
wounds, infected burns, and so on that are caused by Gram-negative microorganisms which
are sensitive to such antibiotics. Synonyms of this drug are carindapen, pyopen, geopen,
gripenin, and others. | [Manufacturing Process]
The required monobenzyl phenylmalonate, MP 68°C, was prepared by treating
a mixture of phenylmalonic acid (18 g) and benzyl alcohol (13 g) in carbon
tetrachloride (80 ml) with dry hydrogen chloride.
Monobenzyl phenylmalonate (13.3 g) in dry benzene (100 ml) was refluxed with thionyl chloride (6.45 g) for 90 minutes, then concentrated in vacuo. The
residual oil was dissolved in dry acetone (50 ml) and added to a stirred, ice-cooled solution of 6-aminopenicillanic acid (9.7 g) in N sodium bicarbonate
solution (135 ml), water (150 ml), and acetone (300 ml). The mixture was
stirred for 30 minutes at 0°C and then for 90 minutes at room temperature,
then concentrated under reduced pressure to remove acetone. The aqueous
solution was brought to pH 2 with dilute hydrochloric acid and extracted with
ether (3 x 100 ml). The ether solution was washed with water and then itself
extracted with sufficient N sodium bicarbonate solution to give an aqueous
phase of pH 7.5. The aqueous layer was separated and evaporated at low
temperature and pressure to leave the impure sodium salt of alpha-
(benzyloxycarbonyl) benzylpenicillin.
This crude product (15.8 g) in water (360 ml) was added to a
prehydrogenated suspension of 10% palladium on charcoal (4 g) in water
(400 ml), and hydrogenation was continued for 30 minutes. The catalyst was
removed and the filtrate was adjusted to pH 7.5 with sodium bicarbonate,
then evaporated at low temperature and pressure. The residue was purified by
chromatography on a column of cellulose powder, eluting first with
butanol/ethanol/water mixture and then with acetone/isopropanol/water. The
main fraction was evaporated at low temperature and pressure to give a 32%
yield of the sodium salt of alpha-carboxybenzylpenicillin as a white powder.
The product was estimated by monometric assay with penicillinase to be 58%
pure. | [Brand name]
Geopen
(Roerig); Pyopen (GlaxoSmithKline). | [Therapeutic Function]
Antibacterial | [Antimicrobial activity]
α-Carboxybenzylpenicillin; the first antipseudomonal penicillin
to be developed. A semisynthetic carboxypenicillin supplied
as the disodium salt for parenteral administration. The two
esterified prodrug formulations, carindacillin (carbenicillin
indanyl sodium) and carfecillin (carbenicillin carboxyphenyl
ester) are no longer available.
It is the least active of the group 5 agents, even against
Ps. aeruginosa (MIC 64 mg/L) with notably reduced activity
against Gram-positive cocci. It is labile to many plasmidmediated
β-lactamases, but is comparatively stable to class C
chromosomal β-lactamases (pp. 228–230). Synergy is demonstrable
with aminoglycosides against Ps. aeruginosa and other
Gram-negative bacteria.
It is not orally absorbed, except in esterified form. A 1 g
intramuscular injection achieves a plasma peak concentration
of 20–30 mg/L after 0.5–1.5 h. The half-life is around 1 h.
Plasma protein binding is 50–60%.
The drug is distributed in the extracellular fluid, providing
concentrations up to 60% of those of the plasma. In patients
with cystic fibrosis sputum concentrations may not reach
inhibitory levels for Ps. aeruginosa. It does not cross the normal
meninges but levels of up to 50% of those of the plasma
can be found in patients with meningitis. Around 80% of the dose appears as unchanged drug in the urine, producing very
high levels (2–4 g/L). It is more rapidly disposed of in patients
with cystic fibrosis.
Hypersensitivity reactions may occur, but these are less
frequent and severe than those associated with benzylpenicillin.
High blood levels sometimes cause a coagulation defect
that has occasionally progressed to life-threatening bleeding
in patients with impaired excretion while receiving 500 mg/kg
per day or more. Reversible abnormalities of liver function
apparently occur more commonly than with other antipseudomonal
penicillins. Since large doses of the drug have
to be used, convulsions can occur (as with other penicillins;
p. 203) and, being administered as the disodium salt, electrolyte
disturbances can result. It was formerly
used for treatment
of serious infections, especially those involving Ps. aeruginosa.
It has extremely limited availability. | [Biological Activity]
carbenicillin is broad-spectrum semisynthetic penicillin derivative used parenterally. | [Synthesis]
Carbenicillin, [2S-(2|á,5|á,6|?)]-3,3-dimethyl-7-oxo-6-(2-carboxy-2-
phenylacetamido)-4-thia-1-azabicyclo[3.2.0]-heptan-2-carboxylic acid (32.1.1.32), is syn�thesized by direct acylation of 6-APA in the presence of sodium bicarbonate by
phenylmalonic acid monobenzyl ester chloride, which forms the benzyl ester of carbeni�cillin (32.1.1.31), the hydrogenolysis of which using palladium on carbon or calcium car�bonate as catalyst gives the desired product (32.1.1.32).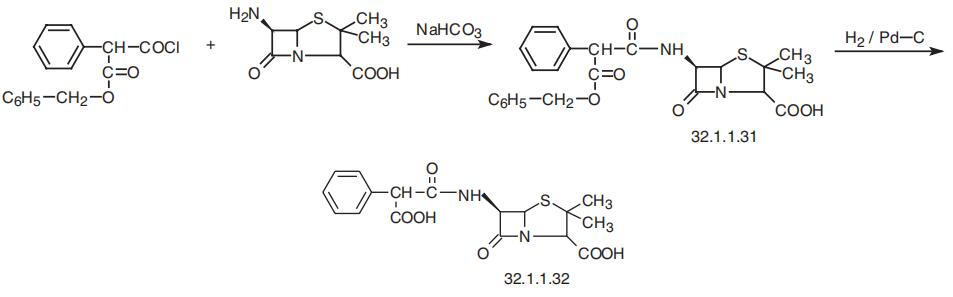
| [storage]
Store at -20°C |
|
| Company Name: |
Musechem
|
| Tel: |
+1-800-259-7612 |
| Website: |
www.musechem.com |
|