| Identification | Back Directory | [Name]
Vortioxetine | [CAS]
508233-74-7 | [Synonyms]
Vortioxetin
Lu AA 21004
Vortioxetine
Vortioxetine, >=99%
Vortioxetine(Lu AA21004)
Trintellix (vortioxetine)
1-(2-((2,4-DiMethylphenyl)thio)phenyl)piperazine
1-[2-(2,4-Dimethylphenylsulfanyl)phenyl]piperazine
1-(2-((2,4-DIMETHYLPHENYL)THIO)PHENYL)PIPERAZINE-HCL
Vortioxetine 1-(2-((2,4-Dimethylphenyl)thio)phenyl)piperazine | [EINECS(EC#)]
1308068-626-2 | [Molecular Formula]
C18H22N2S | [MDL Number]
MFCD22380814 | [MOL File]
508233-74-7.mol | [Molecular Weight]
298.446 |
| Chemical Properties | Back Directory | [Melting point ]
115-117°C | [Boiling point ]
424.8±45.0 °C(Predicted) | [density ]
1.16 | [storage temp. ]
-20°C Freezer | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
8.85±0.10(Predicted) | [color ]
White to Off-White | [InChI]
InChI=1S/C18H22N2S/c1-14-7-8-17(15(2)13-14)21-18-6-4-3-5-16(18)20-11-9-19-10-12-20/h3-8,13,19H,9-12H2,1-2H3 | [InChIKey]
YQNWZWMKLDQSAC-UHFFFAOYSA-N | [SMILES]
N1(C2=CC=CC=C2SC2=CC=C(C)C=C2C)CCNCC1 |
| Hazard Information | Back Directory | [Definition]
ChEBI: An N-arylpiperazine in which the aryl group is specified as 2-[(2,4-dimethylphenyl)sulfanyl]phenyl. Used (as its hydrobromide salt) for treatment of major depressive disorder. | [Description]
In September 2013, vortioxetine (also known as Lu AA21004) was approved in the United States for the treatment of major depressive disorder (MDD). Vortioxetine was discovered from a designed multiple ligand approach to identifying an antidepressant agent that combined SERT inhibition with 5-HT1A agonism to more rapidly desensitize 5-HT1A receptors and 5-HT3A antagonism to improve mood and cognitive function. Vortioxetine has a human SERT IC50=5.4 nM, an EC50=200 nM as a human 5-HT1A receptor agonist (efficacy=96%; Ki=39 nM), and an IC50=12 nM as a human 5-HT3A receptor antagonist (Ki=3.7 nM). It has weak inhibition of the dopamine and norepinephrine transporters, but high affinity for the human β1-noradrenergic receptor (Ki=46 nM), human 5-HT1B receptor (Ki=33 nM, partial agonist), and the human 5-HT-7 receptor (Ki=19 nM, antagonist). | [Originator]
Lundbeck (Denmark) | [History]
Vortioxetine was invented by scientists at Lundbeck who reported the rationale and synthesis for the drug (then called Lu AA21004) in a 2011 paper. Takeda and Lundbeck Announce the U.S. Submission of a New Drug Application for Vortioxetine, an Investigational Drug for the Treatment of Major Depressive Disorder on Oct 1, 2012. Vortioxetine, which was approved by the Food and Drug Administration (FDA) on September 30, 2013, for treatment of major depressive disorder (MDD) , and Brintellix (vortioxetine) Renamed Trintellix (vortioxetine) in U.S. to Avoid Name Confusion. | [Uses]
Vortioxetine can be used in biological study of effectiveness of long term vortioxetine treatment of patients with major depressive disorder. | [Brand name]
Brintellix | [Clinical Use]
Antidepressant | [Synthesis]
The sequence involves iterative palladium-catalyzed carbon¨C
heteroatom bond formations, the first establishing the thioethereal
bond between commercially available thiol (213) and o-iodobromobenzene
(214) employing conditions described by Schopfer
and Schlapbach. Next, Buchwald¨CHartwig conditions were
employed to establish the piperazine linkage, and this was
followed by subjection to warm hydrobromic acid to furnish
vortioxetine hydrobromide (XXVI) in 75% yield across the entire
three-step sequence.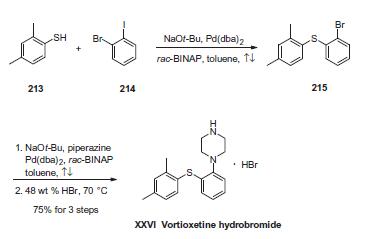
| [in vitro]
vortioxetine (lu aa21004) was the lead compound, displaying high affinity for recombinant human 5-ht1a (ki = 15 nm), 5-ht1b (ki = 33 nm), 5-ht3a (ki = 3.7 nm), 5-ht7 (ki = 19 nm), and noradrenergic β1 (ki = 46 nm) receptors, and sert (ki = 1.6 nm). vortioxetine displayed antagonistic properties at 5-ht3a and 5-ht7 receptors, partial agonist properties at 5-ht1b receptors, agonistic properties at 5-ht1a receptors, and potent inhibition of sert [1]. | [in vivo]
in conscious rats, vortioxetine significantly increased extracellular 5-ht levels in the brain after acute and 3 days of treatment. following the 3-day treatment (5 or 10 mg/kg/day) sert occupancies were only 43% and 57%, respectively [1]. | [target]
5-HT3 receptor | [Drug interactions]
Potentially hazardous interactions with other drugs
Antibacterials: avoid with linezolid; concentration
reduced by rifampicin - consider increasing
vortioxetine dose.
Antidepressants: possible increased risk of
convulsions with SSRIs and, tricyclics; avoid with
moclobemide; increased risk of hypertension and
CNS excitation with MAOIs - avoid.
Antiepileptics: concentration possibly reduced by
carbamazepine, fosphenytoin and phenytoin -
consider increasing vortioxetine dose.
Antimalarials: possible increased risk of convulsions
with mefloquine; avoid with artemether with
lumefantrine and artenimol with piperaquine.
Antipsychotics: possible increased risk of convulsions
with butyrophenones, phenothiazines and
thioxanthenes.
Dopaminergics: risk of CNS excitation and
hypertension with rasagiline and selegiline. | [Metabolism]
Vortioxetine is extensively metabolised in the liver, mainly
by oxidation catalysed by CYP2D6 and to a minor extent
CYP3A4/5 and CYP2C9, and subsequent glucuronic
acid conjugation. The major metabolite of vortioxetine is
pharmacologically inactive.
Approximately two thirds of the inactive vortioxetine
metabolites are excreted in the urine and approximately
one third in the faeces. Only negligible amounts of
vortioxetine are excreted in the faeces. | [References]
[1] bang-andersen b, ruhland t, j?rgensen m, smith g, frederiksen k, jensen kg, zhong h, nielsen sm, hogg s, m?rk a, stensb?l tb. discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (lu aa21004): a novel multimodal compound for the treatment of major depressive disorder. j med chem. 2011;54(9):3206-21.
[2] theunissen el, street d, h?jer am, vermeeren a, van oers a, ramaekers jg. a randomized trial on the acute and steady-state effects of a new antidepressant, vortioxetine (lu aa21004), on actual driving and cognition. clin pharmacol ther. 2013;93(6):493-501. |
| Questions And Answer | Back Directory | [Curative for Major Depression Disorder]
Vortioxetine is a new drug for treating depression, jointly developed and researched by the Danish Lundbeck (Lundbeck) and Japanese Takeda. In September 30, 2013, It is approved by the U.S. Food and Drug Administration (FDA) to enter into market with the brand name of Brintellix, for Major Depression Disorder (MDD) treatment.
In October 2013, the European Medicines Agency (EMA)’s subordinate agency: Committee for Medicinal Products for Human Use (CHMP) recommended that vortioxetine for the treatment of severe depression should be licensed in the European market and be launched into the European market in January 2014.
MDD features mood changes and a series of other symptoms, which have a great impact on the patient's ability to work, sleep, study, eat and enjoy the current happiness. Depressive symptoms can recur many times in life, but some patients may experience only once.
Vortioxetine, mainly by increasing the concentration of serotonin (5-HT) in the central nervous system (CNS), exerts antidepressant effects. Compared with other selective serotonin reuptake inhibitors (SSRIS) or serotonin-norepinephrine reuptake inhibitors (SNRIS), vortioxetine almost has no effect on norepinephrine and dopamine neurons. A number of clinical trials have demonstrated the efficacy, safety and tolerability of vortioxetine in the treatment of MDD.
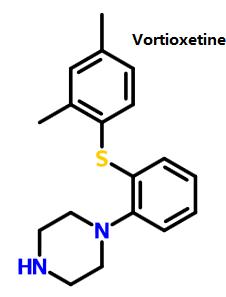
Figure 1 shows the structure of vortioxetine. | [Mechanism of Action]
Vortioxetine is a small molecule piperazine sulfide. WHO w classifies it as antidepressants (N06A). The drug classification system of European Pharmaceutical Market Research Association (EPhMRA) classifies it as hypnotic / sedative drugs (N5B), antidepressants and mood stabilizers (N6A). Currently, depression is thought to be associated with a decrease in 5-HT activity, and decreased activity of norepinephrine (NE) and dopamine (DA) is thought to be associated with depression.
Vortioxetine is a potent serotonin reuptake inhibitor. In human body it has high affinity with serotonin trans-porter (Ki=1.6 nmol - L - 1). But it almost has no affinity with norepinephrine transporter (Ki=113nmol - L-1) and dopamine transporter (Ki= 1000nmol - L - 1). At the same time, it is also a 5-HT1A receptor agonist and 5-HT1B receptor partial agonist, 5-HT1D, 5-HT3 and 5-HT7 receptor antagonist and 5-HT uptake inhibitor. Animal studies have shown that in rats, vinpocetine through interaction on these receptors, will increase depression related brain regions -- the level of the ventral hippocampus, prefrontal cortex extracellular serotonin , dopamine, norepinephrine, acetylcholine and histamine, at the same time it is also regulating the function of y -GABA (γ-aminobutyric acid) and glutamatergic neuron, thus exerting the effect of antidepressant.
| [Pharmacokinetics]
The relative molecular weight of vortioxetine is small, and the plasma protein binding rate is 98 percent, which is not related to plasma concentration. It is widely distributed outside the cell and the apparent distribution volume is about 2600 L. Daily oral administration is 2.5 ~ 60mg, showing the linear pharmacokinetic characteristics in proportion to dosage of administration. Bioavailability is 75 percent and generally after 2 weeks of taking vortioxetine, its concentration will be stable in blood. Cmax is 7 to 11 hours. Vortioxetine is metabolized mainly by oxidation and glucuronidation . After a single oral administration of the radioisotope labeled vortioxetine, about 59 percent of it is excreted through the urine, and about 26 percent were excreted through the feces. There was almost no original vortioxetine 48 hours after administration of the drug. The complete metabolism in body requires about 66 hours. The oxidation is mainly completed through cytochrome P450 enzyme (CYP). The involved CYP includes CYP3A4/5, CYP2C19, CYP2C9, CYP2A6, CYP2C8, CYP2B6 and CYP2D6. Among them, CYP2D6 is the key enzyme which catalyzes and produces the main metabolite of duloxetine carboxylic acid. The drug concentration of CYP2D6 in the plasma with slow metabolism is twice higher than that of CYP2D6 in the plasma with the fast metabolism.
| [Drug Interaction]
- In the different experiment and researches using healthy volunteers as subjects, vortioxetine will be co-administered respectively with CYP2D6 inhibitor bupropion, CYP2C9/CYP2C19/CYP3A inhibitor fluconazole or CYP3A/ permeability glycoprotein (P-gp) inhibitor ketoconazole. The vortioxetine’s bioavailability will increase. Therefore when vortioxetine and strong CYP2D6 inhibitors (such as bupropion, fluoxetine, paroxetine and quinidine) are co-administered, the dosage should be halved.
- When vortioxetine and strong CYP inducers (rifampicin) are co-administered, its bioavailability will be reduced. Therefore when vortioxetine is co-administered with rifampicin or other strong CYP inducers (such as carbamazepine, Phenytoin sodium), the dosage should be increased, but the maximum dosage should not be three times higher than the normal dose.
- In addition, the use of 5-HT drugs itself is a risk factors of incurring abnormal bleeding. Therefore when vortioxetine is co-administered with aspirin, nonsteroidal anti-inflammatory drugs or other drugs affecting blood coagulation, special attention should be drawn to the abnormal bleeding of patients.
- Considering the functional mechanism of vortioxetine and potential toxicity of serotonin, so when it is co-administered with other drugs affecting 5-HT neurotransmitter systems (such as SSRIs, SNRIs, and triptans, buspirone, tramadol and tryptophan products), lt is likely to occur 5-HT syndrome. Therefore, close attention should be paid to the risk of 5-HT syndrome when it is used in combination with such drug. Once the 5-HT syndrome occurs, all such drugs should be discontinued immediately.
- Similar to the above reasons, monoamine oxidase inhibitors (MAOIs) are prohibited from being used in combination with MAOIs. MAOIs can not be administered within 21d after discontinuation of vortioxetine, and vortioxetine can not be administered within 14d after discontinuation of MAOIs. Because the combination of both will increase the risk of 5-HT syndrome in patients.
| [Synthesis]
- 2,4-dimethyl-1- iodo-benzene reacts with 2- bromophenyl thiophenol and Bis(dibenzylidene acetone)palladium, under sodium tert-butoxide’s catalysis to generate vortioxetine
- 2,4- dimethyl thiophenol and 2- bromo benzene and piperazine(1-BOC-piperazine) react under the catalysis of Bis(dibenzylidene acetone)palladium , 1, 1'- linked naphthalene -2, 2'- bis diphenyl phosphine and sodium tert-butoxide to generate vortioxetine.
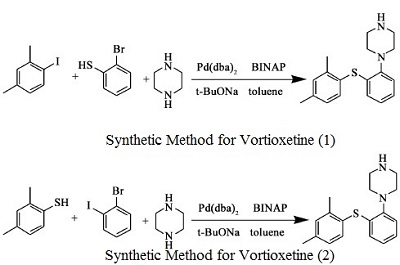 Figure 1 is a chemical reaction diagram for the synthesis of vortioxetine.
Figure 1 is a chemical reaction diagram for the synthesis of vortioxetine.
|
|
|