| Identification | More | [Name]
ALDOSTERONE | [CAS]
52-39-1 | [Synonyms]
11B-21-DIHYDROXY-3,20-DIOXO-4-PREGNAN-18-AL
(11BETA)-11,21-DIHYDROXY-3,20-DIOXO-PREGN-4-EN-18-AL
11-BETA,18-EPOXY-18,21-DIHYDROXY-4-PREGNENE-3,20-DIONE
11BETA,21-DIHYDROXY-3,20-DIOXO-4-PREGNEN-18-AL
11BETA,21-DIHYDROXYPREGN-4-ENE-3,18,20-TRIONE
18-ALDOCORTICOSTERONE
18-KETOCORTICOSTERONE
4-PREGNEN-11-BETA, 21-DIOL-3,18,20-TRIONE
4-PREGNEN-11-BETA,21-DIOL-3,20-DIONE 18-AL
4-PREGNEN-18-AL-11B,21-DIOL-3,20-DIONE
4-PREGNEN-18-AL-11-BETA,21-DIOL-3,20-DIONE
ALDOSTERONE
ELECTROCORTIN
LECTROCORTIN
REICHSTEIN X
11,21-Dihydroxy-3,20-dioxopregn-4-en-18-al
11beta,21-Dihydroxy-3,20-dioxo-pregn-4-en-18-al
18-Oxocorticosterone
21-dihydroxy-3,20-dioxo-1(11-beta)-pregn-4-en-18-a
21-dihydroxy-3,20-dioxo-1(11beta)-pregn-4-en-18-a | [EINECS(EC#)]
200-139-9 | [Molecular Formula]
C21H28O5 | [MDL Number]
MFCD00051136 | [Molecular Weight]
360.44 | [MOL File]
52-39-1.mol |
| Chemical Properties | Back Directory | [Appearance]
White Solid | [Melting point ]
170-172°C | [alpha ]
D23 +152.2° (anhydr; c = 2 in acetone); D25 +161° (c = 0.1 in chloroform) | [Boiling point ]
412.46°C (rough estimate) | [density ]
1.28 | [refractive index ]
1.6120 (estimate) | [Fp ]
2℃ | [storage temp. ]
2-8°C
| [solubility ]
Acetone (Slightly), Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | [form ]
Crystalline Powder | [pka]
12.98±0.10(Predicted) | [color ]
White | [Optical Rotation]
+14523 (c 0.99, acetone) | [Water Solubility ]
51.18mg/L(37 ºC) | [Usage]
An adrenocortical steroid which exerts regulatory influence on metabolism of electrolytes and water. Biologically active aldosterone isomer; a mineralocorticoid produced by the adrenal cortex that induces urinary excretion of K+ and renal reabsorpti | [Merck ]
13,224 | [BRN ]
3224996 | [Major Application]
clinical testing
clinical testing | [InChIKey]
PQSUYGKTWSAVDQ-ZVIOFETBSA-N | [SMILES]
C[C@]12CCC(=O)C=C1CC[C@H]3[C@@H]4CC[C@H](C(=O)CO)[C@]4(C[C@H](O)[C@H]23)C=O | [CAS DataBase Reference]
52-39-1(CAS DataBase Reference) | [EPA Substance Registry System]
Aldosterone (52-39-1) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
UN 1648 3 / PGII | [WGK Germany ]
3
| [RTECS ]
TU4523000
| [F ]
22-24-25 | [TSCA ]
TSCA listed | [HS Code ]
29372900 | [Storage Class]
3 - Flammable liquids | [Hazard Classifications]
Acute Tox. 4 Dermal
Acute Tox. 4 Inhalation
Acute Tox. 4 Oral
Eye Irrit. 2
Flam. Liq. 2 |
| Hazard Information | Back Directory | [Description]
Aldosterone is a mineralocorticosteroid that takes part in the regulation of electrolytic balance
in the organism. Aldosterone lowers excretion of sodium ions from the body, thus
increasing their reabsorption and increasing secretion of potassium ions in renal tubules.
Being a competitive antagonist of aldosterone, spironlactone blocks aldosterone receptors,
thus increasing excretion of sodium, chloride, and corresponding equivalents of water with
urine, thus retaining the amount of potassium ions in the organism. Spironolactone is used
both individually as well as in combination with thiazides, since it lowers kaliuresis caused
by thiazide diuretics. | [Chemical Properties]
White Solid | [Originator]
Aldosterone ,Sigma Chemical
Company | [Uses]
A labelled adrenocortical steroid which exerts regulatory influence on metabolism of electrolytes and water. Biologically active aldosterone isomer; a mineralocorticoid produced by the adrenal cortex that induces urinary excretion of K+ and renal re | [Uses]
hypnotic, anastethic | [Uses]
Imatinib intermediate | [Uses]
Labelled Aldosterone. Aldosterone is an adrenocortical steroid which exerts regulatory influence on metabolism of electrolytes and water. Biologically active aldosterone isomer; a mineralocorticoid pr
oduced by the adrenal cortex that induces urinary excretion of K+ and renal reabsorptioon of Na+. Exists as an equilibrium mixture of the aldehyde and the hemiacetal. | [Uses]
The d-isomer of aldosterone is considered to be the biologically active isomer. | [Definition]
aldosterone: A hormone producedby the adrenal glands that controlsexcretion of sodium by the kidneysand thereby maintains the balance ofsalt and water in the body fluids. | [Definition]
ChEBI: A pregnane-based steroidal hormone produced by the outer-section (zona glomerulosa) of the adrenal cortex in the adrenal gland, and acts on the distal tubules and collecting ducts of the kidney to cause the conservation of sodium, secretion of potassium, i
creased water retention, and increased blood pressure. The overall effect of aldosterone is to increase reabsorption of ions and water in the kidney. | [Therapeutic Function]
Mineralocorticoid | [Biological Functions]
Aldosterone, produced by the adrenal cortex, acts at epithelial
cells in the distal tubule of the nephron to increase
the reabsorption of sodium and is therefore considered an important hormone in the regulation of electrolyte
balance. Aldosterone exerts its effects at the
nephron through mineralocorticoid receptors, which
translocate to the nucleus upon aldosterone binding
and exert genomic effects leading to increased sodium
reabsorption. In addition to the epithelial effects of aldosterone
at mineralocorticoid receptors, nonepithelial
cells, including cardiac muscle and vascular smooth
muscle cells and cells in the brain, can respond to aldosterone
and result in left ventricular hypertrophy,
cardiac and vascular fibrosis, and stimulation of sympathetic
nervous system activity.
Spironolactone (Aldactone), an antagonist of the aldosterone
mineralocorticoid receptor, is used to treat primary
aldosteronism, essential hypertension, and congestive
heart failure. In the treatment of
hypertension resulting from adrenal adenoma (primary aldosteronism)
and in patients with essential hypertension,
spironolactone lowers blood pressure primarily through
blockade of epithelial mineralocorticoid receptors in the
kidney, reductions in sodium and water reabsorption, and
diuresis.The use of spironolactone in the treatment of essential
hypertension is typically restricted to patients who
do not respond appropriately to other agents and is often
used in combination drug therapy. In large-scale clinical
trials in patients with severe heart failure, administration
of spironolactone markedly reduced morbidity and mortality
without reducing blood pressure. Spironolactone is
used to treat patients with moderate to severe heart failure
who exhibit symptoms and ventricular dysfunction despite
treatment with an ACE inhibitor or a diuretic.
Adverse effects of spironolactone therapy include
hyperkalemia, gastrointestinal problems, gynecomastia
(breast enlargement in males), and impotence. Gynecomastia
and impotence arising from spironolactone
treatment are results of significant blockade of the androgen
and mineralocorticoid receptors.Novel selective
mineralocorticoid receptor antagonists, such as eplerenone,
are in clinical trials. | [Health Hazard]
Aldosterone(Aldocortin; electrocortin; mineralocorticoid; 18-oxocorticosterone): (1)Maintenance of normal electrolyte blood balances;(2)Prolongs survival of adrenalectomized animals;(3)Accelerates gluconeogenesis;(4)Regulates kidney function. | [Synthesis]
Aldosterone, 11|?,21-dihydroxypregn-4-en-2,18,20-trione (27.2.4), is synthesized
from 21-O-acetylcorticosterone, which when reacted with nitrosyl chloride in
pyridine gives the nitrite 27.2.1. When photochemically irradiated, this compound is transformed
to the oxime 27.2.2, which is hydrolyzed by nitrous acid and forms the semiacetal
27.2.3, which is an acetate of the desired aldosterone. Alkaline hydrolysis of the acetyl
group of this compound leads to the desired aldosterone (27.2.4) .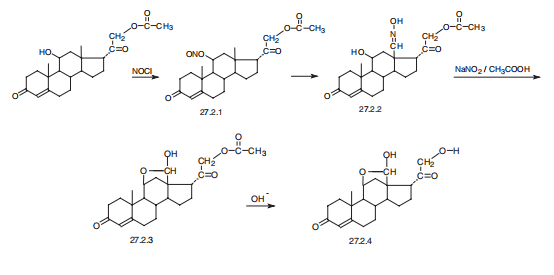
| [Purification Methods]
Crystallise aldosterone from aqueous acetone. It exists in solution as an equilibrium mixture of free aldehyde and its cyclic hemiacetal, favouring the hemiacetal. The 21-acetate crystallises from Me2CO/Et2O or CH2Cl2/EtOAc and has m 198-199o, [�] D +121.7o (c 0.7, CHCl3). [Barton et al. J Chem Soc Perkin Trans 1 2243 1975, Beilstein 8 IV 3491.] |
|
|