| Identification | Back Directory | [Name]
METARAMINOL | [CAS]
54-49-9 | [Synonyms]
C07146
icoralb
pressonex
pressorol
metaradrine
METARAMINOL
1-metaraminol
l-metaraminol
m-hydroxypropadrine
hydroxynorephedrine
m-hydroxynorephedrine
(-)-erythro-metaraminol
Metaraminol (free base)
NOREPHEDRINE, M-HYDROXY-
m-hydroxyphenylpropanolamine
Norephedrine, m-hydroxy- (6CI)
3-hydroxyphenylisopropanolamine
Metaraminol Bitartrate 33402-03-8 /
-(m-hydroxyphenyl)-2-amino-1-propanol
2-amino-1-(m-hydroxyphenyl)-1-propanol
1-(m-hydroxyphenyl)-2-amino-1-propanol
(1-Aminoethyl)-3-hydroxybenzenemethanol
alpha-(m-hydroxyphenyl)-beta-aminopropanol
alpha-(1-aminoethyl)-m-hydroxybenzylalcohol
m-hydroxy-alpha-(1-aminoethyl)-benzylalcohol
alpha-(1-aminoethyl)-3-hydroxybenzenemethanol
1-alpha-(1-aminoethyl)-m-hydroxybenzylalcohol
alpha-(1-aminoethyl)-m-hydroxy,(-)-benzylalcoho
(-)-(1R,2S)-1-(m-Hydroxyphenyl)-2-amino-1-propanol
Benzenemethanol, a-[(1S)-1-aminoethyl]-3-hydroxy-,(aR)-
Benzyl alcohol, a-(1-aminoethyl)-m-hydroxy-, (-)- (8CI)
BENZENEMETHANOL, ALPHA-[(1S)-1-(AMINO)ETHYL]-3-HYDROXY-
alpha-(1-aminoethyl)-3-hydroxy-,(r-(r*,s*))-benzenemethano
Benzenemethanol, a-(1-aminoethyl)-3-hydroxy-, [R-(R*,S*)]-
Benzenemethanol, a-[(1S)-1-aminoethyl]-3-hydroxy-, (aR)- (9CI) | [Molecular Formula]
C9H13NO2 | [MDL Number]
MFCD01664455 | [MOL File]
54-49-9.mol | [Molecular Weight]
167.21 |
| Chemical Properties | Back Directory | [Appearance]
Colourless solid | [Melting point ]
107.5°C | [Boiling point ]
295.79°C (rough estimate) | [density ]
1.1222 (rough estimate) | [refractive index ]
1.4760 (estimate) | [pka]
pKa 8.6 (Uncertain) |
| Hazard Information | Back Directory | [Chemical Properties]
Colourless solid | [Definition]
ChEBI: A member of the class of phenylethanolamines that is 2-amino-1-phenylethanol substituted by a methyl group at position 2 and a phenolic hydroxy group at position 1. A sympathomimetic agent , it is used in the treatment of hypotension. | [Originator]
Aramine,MSD, US,1952 | [Uses]
Adrenergic. | [Uses]
Metaraminol is a direct and indirect non-specific adrenoceptor agonist. It
acts primarily via α1-receptors, causing vasoconstriction with subsequent
increase in arterial pressure and reflex bradycardia. It is administered via i.v.
bolus injection at a dose of 0.5–2 mg, titrated to effect. | [Uses]
Metaraminol is a sympathomimetic amine of both direct and indirect action that has hemo�dynamic characteristics similar to norepinephrine. It has the ability to elevate both systolic
and diastolic blood pressure.
It is used in hypotensive shock for the purpose of elevating blood pressure, which can
result from spinal anesthesia, surgical complications, and head trauma. | [Manufacturing Process]
The hydrochloride of the m-hydroxyphenylpropanolamine may be prepared by
dissolving or suspending 90 parts of m-hydroxyphenylethyl ketone, O =
C(C6H4-OH)-C2H5, in about 400 parts of ether. Hydrogen chloride is slowly
bubbled through the solution or suspension while agitating it and 61.8 g of
butyl nitrite is added during the course of 60 to 90 minutes. During the
addition of the butyl nitrite the suspended m-hydroxyphenylethyl ketone
gradually dissolves. The mixture or solution is allowed to stand for at least an
hour, but preferably overnight. It is then repeatedly extracted with dilute alkali
until all alkali-soluble material is removed. The alkaline extract is slowly
acidified and the precipitate which forms is crude m-hydroxyphenyl-αoximinoethyl ketone. After recrystallization from water this melts at 138°C.
10.8 parts of the meta ketone is dissolved in about 125 parts of absolute
alcohol containing 5.6 parts of hydrogen chloride. The solution is agitated with
a catalyst such as the palladium catalyst above described in an atmsophere of hydrogen until no more hydrogen is absorbed. This requires from 60 to 90
minutes or more. When reduction is complete the catalyst is filtered off and
the filtrate evaporated to dryness by being placed in a desiccator at ordinary
temperature.
The residue is the hydrochloride of m-hydroxyphenyl-α-aminoethyl ketone.
This is purified by recrystallization from absolute alcohol. It is then dissolved
in 200 parts of water and agitated with a further quantity of the palladium
catalyst in an atmosphere of hydrogen until saturated. The product thus
recovered from the solution is the hydrochloride of m-hydroxyphenylpropanol
amine. After recrystallization from absolute alcohol this melts at 177°C. The
corresponding free base can be prepared from the hydrochloride by treatment
with ammonia, according to US Patent 1,995,709.
Metaraminol is often used in the form of the bitartrate. | [Brand name]
Aramine (Merck)
. | [Therapeutic Function]
Hypertensive | [General Description]
Metaraminol is the N-desmethyl- -methylanalog of phenylephrine. It possesses a mixed mechanismof action, with its direct-acting effects mainly on 1-receptors. It is used parenterally as a vasopressor in thetreatment and prevention of the acute hypotensive stateoccurring with spinal anesthesia. It also has been used totreat severe hypotension brought on by other traumas thatinduce shock. | [Clinical Use]
#N/A | [Synthesis]
Metaraminol, L-1-(3-hydroxyphenyl)-2-aminopropan-1-ol (11.3.11), is synthesized in two ways. The first way is synthetic, and it is from 3-hydroxypropiophenone.
The hydroxyl group is protected by alkylation with benzyl chloride, giving 3-benzy�loxypropiophenone (11.3.8). Upon reaction with butylnitrite, it undergoes nitrosation into
the isonitrosoketone (11.3.9), which by reduction using semisynthetic, consisting of fermentation of D-glucose in the presence
of 3-acetoxybenzaldehyde, which forms (-)-1-hydroxy-1-(3-hydroxyphenyl)-acetone
(11.3.12), the carbonyl group of which is reduced by hydrogen over a palladium catalyst
in the presence of ammonia, giving metaraminol (11.3.11) [62¨C65].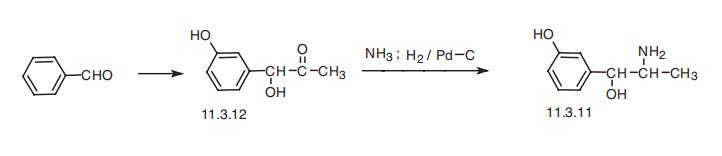
| [Drug interactions]
Potentially hazardous interactions with other drugs
Adrenergic neurone blockers: hypotensive effect
antagonised.
Anaesthetics: risk of ventricular arrhythmias with
isoflurane - avoid.
Antibacterials: risk of hypertensive crisis with
linezolid and tedizolid - avoid for at least 2 weeks
after stopping linezolid and tedizolid.
Antidepressants: risk of hypertensive crisis with
MAOIs and moclobemide - avoid for at least 2
weeks after stopping MAOIs.
Dopaminergics: avoid with rasagiline and selegiline | [Metabolism]
Hepatically metabolised. |
|
| Company Name: |
BOYAN PHARMA Gold
|
| Tel: |
18566078841 18566078841 |
| Website: |
boyanpharma.com/index.php?c=category&id=9 |
|