| Identification | More | [Name]
Ergosterol | [CAS]
57-87-4 | [Synonyms]
(22E)-Ergosta-5,7,22-trien-3beta-ol
24-METHYLCHLOLESTA-5,7,22-TRIEN-3BETA-OL
(3BETA,22E)-ERGOSTA-5,7,22-TRIEN-3-OL
3BETA-HYDROXY-5,7,22-ERGOSTATRIENE
5,7,22-CHOLESTATETRIEN-24BETA-METHYL-3BETA-OL
5,7,22-CHOLESTATRIEN-24-BETA-METHYL-3-BETA-OL
5,7,22-CHOLESTATRIEN-24-METHYL-3-OL
5,7,22-ERGOSTATRIEN-3BETA-OL
ERGOSTERIN
ERGOSTERINE
ERGOSTEROL
PROVITAMIN D2
PROVITAMINE D2
(22E)-Ergosta-5,7,22-trien-3-ol
(22E,24R)-Ergosta-5,7,22-trien-3-ol
(3beta)-Ergosta-5,7,22-trien-3-ol
22-trien-3-ol,(3beta,22e)-ergosta-7
3-Hydroxy-(22E,24R)-ergosta-5,7,22-triene
7,22-trien-3-ol,(3.beta.)-Ergosta-5
delta-5,7,22-ergostatrien-3beta-ol | [EINECS(EC#)]
200-352-7 | [Molecular Formula]
C28H44O | [MDL Number]
MFCD00003623 | [Molecular Weight]
396.65 | [MOL File]
57-87-4.mol |
| Chemical Properties | Back Directory | [Appearance]
solid | [Melting point ]
156-158 °C(lit.)
| [alpha ]
-120 º (c=1, CHC13) | [Boiling point ]
250 °C (1.3 mmHg)
| [density ]
0.9784 (rough estimate) | [refractive index ]
-112.5 ° (C=1, THF) | [storage temp. ]
2-8°C
| [solubility ]
Acetone (Slightly, Heated), Chloroform (Slightly), Ethyl Acetate (Slightly, Heated) | [form ]
Crystalline Powder or Crystalline Needles | [pka]
14.91±0.70(Predicted) | [color ]
White to off-white | [Stability:]
Stable, but may be light or air sensitive. Incompatible with acids, strong oxidizing agents. | [biological source]
plant | [Optical Rotation]
[α]20/D 120±10°, c = 1% in chloroform | [Water Solubility ]
PRACTICALLY INSOLUBLE | [Sensitive ]
Light Sensitive | [Merck ]
14,3659 | [BRN ]
2338604 | [Cosmetics Ingredients Functions]
SKIN CONDITIONING | [InChIKey]
DNVPQKQSNYMLRS-APGDWVJJSA-N | [SMILES]
[H][C@@]1(CC[C@@]2([H])C3=CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)\C=C\[C@H](C)C(C)C | [LogP]
9.300 (est) | [Uses]
Ergosterol is a steroid alcohol that when irradiated with ultraviolet
light yields calciferol (vitamin d2). irradiated ergosterol is added to
milk for vitamin d fortification. | [CAS DataBase Reference]
57-87-4(CAS DataBase Reference) | [NIST Chemistry Reference]
Ergosterol(57-87-4) | [Storage Precautions]
Air sensitive;Light sensitive | [EPA Substance Registry System]
Ergosta-5,7,22-trien-3-ol, (3.beta.,22E)- (57-87-4) |
| Safety Data | Back Directory | [Hazard Codes ]
T+,T | [Risk Statements ]
R28:Very Toxic if swallowed.
R48/20/22:Harmful: danger of serious damage to health by prolonged exposure through inhalation and if swallowed .
R40:Limited evidence of a carcinogenic effect.
R38:Irritating to the skin.
R25:Toxic if swallowed. | [Safety Statements ]
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S36/37:Wear suitable protective clothing and gloves .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 2811 6.1/PG 2
| [WGK Germany ]
3
| [F ]
1-3-8-10 | [HazardClass ]
6.1 | [PackingGroup ]
II | [HS Code ]
29334900 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Aquatic Chronic 4 | [Hazardous Substances Data]
57-87-4(Hazardous Substances Data) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Ribonucleic acid-->Yeast extract-->ZYMOSAN A-->4a,13b-Etheno-1H,9H-benzo[c]cyclopenta[h][1,2,4]triazolo[1,2-a]cinnoline-1,3(2H)-dione, 5,6,7,8,8a,8b,10,10a,11,12,13,13a-dodecahydro-6-hydroxy-8a,10a-dimethyl-2-phenyl-11-[(1R,2E,4R)-1,4,5-trimethyl-2-hexenyl]-, (4aS,6S,8aR,8bR,10aR,11R,13aR,13bS)--->Ergosta-4,7,22-trien-3-one, (22E)- | [Preparation Products]
Vitamin D2-->22(S),23(S)-Homobrassinolide-->Stellasterol |
| Hazard Information | Back Directory | [Hazard]
Due to its ability to catalyze calcium deposition in the bony structure (thus preventing rickets),
overdosage of vitamin D may be harmful. | [Description]
Ergosterol is a membrane component and with few
exceptions is restricted to eumycotic fungi (101). As a
constituent of intact membranes, its abundance should
reflect the amount of living fungal biomass in an
environment. This membrane component has been related
to biomass by a number of investigators and the values
range from 1.9 to 11.5 mg ergosterol g?1 mycelium (102).
These conversion factors yield very high values for fungal
biomass, and seem unrealistic (102) when compared with
independent measures of bacterial biomass. It is likely
that the ergosterol assay detects nonliving hyphae and
these measures may overestimate viable fungal biomass.
Similarly, chitin is a dominant cell wall component in
most fungi and has been proposed as a unique marker
for total fungal biomass. | [Chemical Properties]
solid | [Definition]
ChEBI: A phytosterol consisting of ergostane having double bonds at the 5,6-, 7,8- and 22,23-positions as well as a 3beta-hydroxy group. | [Definition]
ergosterol: A sterol occurring infungi, bacteria, algae, and plants. It isconverted into vitamin D2 by the actionof ultraviolet light. | [General Description]
Ergosterol or provitamin D2 is a biological precursor of Vitamin D2. It is found predominantly in cell membranes of fungi and protists such as trypanosomes. | [Biochem/physiol Actions]
Ergosterol has a role in maintaining the integrity of the cell membrane and its fluidity. The sterol has anti-tumor and anti-inflammatory properties. | [Synthesis]
Ergosterol is an important component of fungal cell membranes and is primarily obtained through industrial fermentation. Yeast strains, such as Saccharomyces cerevisiae, can naturally synthesize this compound via the mevalonate pathway. This biosynthetic pathway involves farnesyl pyrophosphatase-catalyzed conversion to squalene, followed by squalene cyclization to lanosterol, and then a series of subsequent demethylation, oxidation, and desaturation steps to finally produce ergosterol. While total chemical synthesis is useful for research, large-scale production relies on optimizing fermentation conditions and using synthetic biology techniques to modify yeast metabolic pathways to increase yield. | [Purification Methods]
Crystallise ergosterol from EtOAc, then from ethylene dichloride or EtOH/*C6H6 (3:1). It has been purified by conversion to the isobutyl ester which crystallises from Et2O/Me2CO (1:3) with m: turbid at 148o, melts at 159o and becomes clear at 162o, followed by hydrolysis, [Bill & Honeywell J Biol Chem 80 15 1938]. When crystallised from EtOH, it forms the 1.5-hydrate m 168o. The water is difficult to remove giving an amorphous solid m 166-183o, b 250o/high vacuum. It is light sensitive. The benzoate has m 169-171o, after crystallisation from Me2CO/*C6H6 (4:1) after prolonged standing at 0o and becomes highly charged, with [�] D20 -177o (c 1, CHCl3). [UV of sterols: Hogness et al. J Biol Chem 120 239 1937, Beilstein 6 IV 4407.] |
| Questions And Answer | Back Directory | [Overview]
Sterols are essential structural and regulatory components of eukaryotic cell membranes. Mammals, plants and fungi produce similar sterols, which differ in the number and location of double bonds and methyl side chains. Ergosterol (ergosta-5, 7, 22-trien-3β-ol) is a sterol found in fungi, and named for ergot, the common name of members of the fungal genus Claviceps from which ergosterol was first isolated. Ergosterol is the end product of the sterol biosynthetic pathway and is the major sterol in yeasts. Like cholesterol in mammalian cells, it is responsible for membrane fluidity and permeability[6]. Ergosterol is a very important component of yeast and other fungal cell membranes, playing many important roles as that cholesterol plays in animal cells.[1] Its specificity in higher fungi is thought to be related to the climatic instabilities (highly varying humidity and moisture conditions) encountered by these organisms in their typical ecological niches (plant and animal surfaces, soil). Thus, despite the added energy requirements of ergosterol synthesis (if compared to cholesterol), ergosterol is thought to have evolved as a nearly ubiquitous, evolutionarily advantageous fungal alternative to cholesterol.
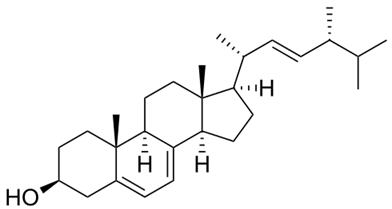
Figure 1 the chemical structure of ergosterol | [A target for antifungal drugs]
Since ergosterol is a key component in cell membranes of fungi, yet absent in those of animals. It has become a very useful target for antifungal drugs. Ergosterol is also present in the cell membranes of some protists, such as trypanosomes. This also becomes the basis for the use of some antifungals against West African sleeping sickness.
Antifungal drugs targeting ergosterol includes Amphotericin B[2, 3], fluconazole, miconazole, itraconazole, and clotrimazole. Amphotericin B acts by binding to sterols (ergosterol) in the cell membrane of susceptible fungi[2, 3]. This creates a transmembrane channel, and the resultant change in membrane permeability allowing leakage of intracellular components. Ergosterol, the principal sterol in the fungal cytoplasmic membrane, is the target site of action of amphotericin B and the azoles. Amphotericin B, a polyene, binds irreversibly to ergosterol, resulting in disruption of membrane integrity and ultimately cell death[2, 3]. Amphotericin B, though has been replaced by safer agents in most circumstances, is still used, despite its side effects, for life-threatening fungal or protozoan infections.
Fluconazole, miconazole, itraconazole, and clotrimazole work in a different way, take effect through inhibiting synthesis of ergosterol from lanosterol by interfering with14α-demethylase[4, 5]. Fluconazole interacts with 14-α demethylase, a cytochrome P-450 enzyme necessary to convert lanosterol to ergosterol. As ergosterol is an essential component of the fungal cell membrane, inhibition of its synthesis results in increased cellular permeability causing leakage of cellular contents. Fluconazole may also inhibit endogenous respiration, interact with membrane phospholipids, inhibit the transformation of yeasts to mycelial forms, inhibit purine uptake, and impair triglyceride and/or phospholipid biosynthesis.
| [Reference]
- Ruzicka, S., Edgerton, D., Norman, M., & Hill, T. (2000). The utility of ergosterol as a bioindicator of fungi in temperate soils. Soil Biology & Biochemistry, 32(7), 989-1005.
- Vertut-Croquin, A, et al. "Differences in the interaction of the polyene antibiotic amphotericin B with cholesterolor ergosterol-containing phospholipid vesicles. A circular dichroism and permeability study." Biochemistry 22.12(1983): 2939-44.
- Baginski, M, H. Resat, and E. Borowski. "Comparative molecular dynamics simulations of amphotericin B-cholesterol/ergosterol membrane channels." Biochim Biophys Acta 1567.1-2(2002): 63-78.
- Ballard, S. A., et al. "A novel method for studying ergosterol biosynthesis by a cell-free preparation of Aspergillus fumigatus and its inhibition by azole antifungal agents." J Med Vet Mycol 28.4(1990):335-344.
- Lv, Quan-Zhen; Yan, Lan; Jiang, Yuan-Ying (2016). "The synthesis, regulation, and functions of sterols in Candida albicans: Well-known but still lots to learn". Virulence. 7 (6): 649–659.
- Parks, L. W., Smith, S. J. & Crowley, J. H. (1995). Biochemical and physiological effects of sterol alterations in yeast – a review. Lipids 30, 227–230.
|
|
|